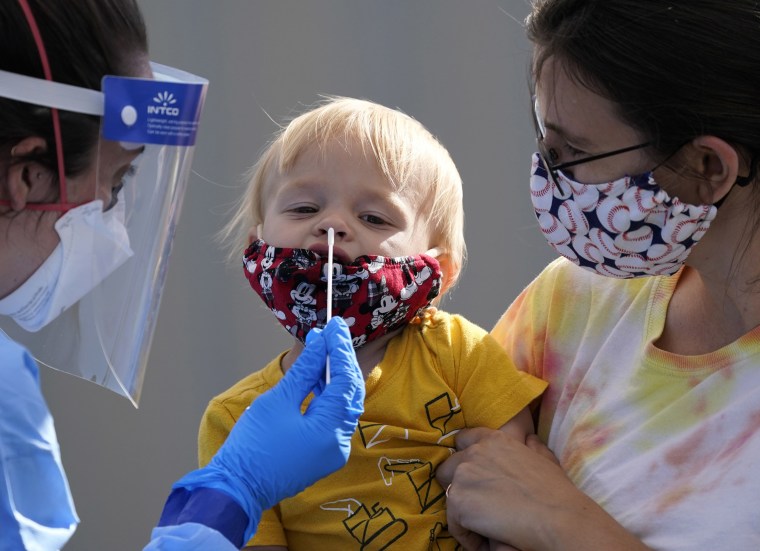
Moderna asked the Food and Drug Administration on Thursday to expand the use of its Covid-19 vaccine to children ages 6 months to 5 years.
The drugmaker's request will now be considered by the FDA, which is expected to make a final decision in June.
Full coverage of the coronavirus outbreak
The agency is expected to seek the advice of its advisory committee, the Vaccines and Related Biological Products Advisory Committee.
Children under 5 are the only group in the U.S. ineligible to receive a Covid vaccine; the Pfizer-BioNTech vaccine is available to anyone as young as 5, while Moderna's and Johnson & Johnson's shots are only available to adults.
Moderna’s vaccine for children 6 months to 5 years old is given as two 25-microgram doses four weeks apart. The dosage is a quarter of the dosage given to adults.
Clinical trial results released in March found the vaccine wasn’t particularly effective against infection in the age group, though it did prevent serious illness.
Moderna chief medical officer Dr. Paul Burton told NBC News that the lower efficacy against infection was due to the extremely contagious omicron variant, which has dealt a blow to the effectiveness of the current vaccines for other age groups as well.
Still, the two-dose vaccine for young children provides a “good level of protection” and “can protect these kids,” Burton said.
Moderna's application to FDA may come as welcome news to parents eager to get their children vaccinated. A Centers for Disease Control and Prevention study published in March found that during the winter omicron surge, children under 5 were hospitalized at about five times the rate they were during the delta wave.
The CDC estimates that, by February, three-quarters of children and teens in the U.S. caught Covid at least once, though agency officials noted it remains unclear how long that protection from those previous infections will last or whether it's as robust as the protection provided by the vaccines.
A vaccine will be an important tool for this age group because vaccination has been shown "to provide more protection than natural infection," said Dr. Sean O’Leary, vice chair of the Committee on Infectious Diseases for the American Academy of Pediatrics.
Dr. Jesse Goodman of Georgetown University, a former FDA vaccine chief, agreed, saying protection from natural infection can "vary tremendously" among individuals.
Pfizer is also preparing to submit data to the FDA on a potential three-dose regimen of its vaccine for children under 5.
Pfizer in February postponed its application for the age group after it found two doses of its vaccine didn't work well in a clinical trial of children ages 2 to 4.
If Pfizer submits its application soon, it's unclear if the agency would authorize the Moderna and Pfizer vaccines one at a time, or hold off and authorize both at the same time.
And while Moderna filed an application for a two-dose regimen, Burton said the company expects to seek authorization for a booster dose for the age group. The shot could be available in the fall, he said, and could be a so-called bivalent vaccine, which targets two strains of the coronavirus in a single shot.
The majority of side effects from the two-dose regimen were “mild or moderate,” according to the company, and no cases of a rare heart inflammation condition called myocarditis were reported.
The risk of myocarditis has been a particular sticking point for the FDA in Moderna’s vaccine application for kids ages 12 to 17. That application remains in limbo.
If the shot for the youngest age group is authorized, federal regulators will need to articulate to parents the benefit of getting the vaccine, including that it can lower the risk of severe illness and lower the risk of MIS-C, or multisystem inflammatory syndrome, Goodman said.
Vaccination rates for older children remain low in the U.S.
Less than 30 percent of children ages 5 to 11 have received two doses of a vaccine, according to data from the CDC.
Follow NBC HEALTH on Twitter & Facebook.
