Researchers have discovered a series of radically different building blocks for new materials, made from tiny spheres snuggled into oddly shaped clusters. Nature seems to have given these spheres an altogether new set of instructions for how small parcels of matter should fit together, a problem researchers have pondered for centuries. The study appears in the journal Science, published by AAAS, the science society. While these findings probably won’t help you stuff your suitcase for summer vacation, they could enhance fiber-optic technology as well as our understanding of the structure of matter.
How the materials around us look, feel, and function is largely a matter of how their components fit together. A sparkling diamond and a lump of graphite, for example, are both made of carbon; the difference is how the carbon atoms are arranged in space.
The clustering behavior discovered by David Pine and his colleagues at the University of California at Santa Barbara was also highly specific.
“If you take ping pong balls and some glue, there is an infinite number of ways you can put together seven spheres,” explained co-author Vinothan Manoharan. “When we look at these structures, they’re always forming the same shape, depending on the number of spheres.”
Scientists have worked for centuries to identify and describe the various patterns that make the “optimal” structures for atoms, molecules or larger units of matter. This field of mathematics is known as sphere packing, since spheres typically stand in for units that are too small to see.
In many substances, such as metals and noble gases, the units are spaced equidistantly along staggered rows, like oranges displayed in a supermarket. While it’s pretty intuitive that this is the most efficient way to pack together a large number of round objects, it has been devilishly hard to prove it mathematically. Johannes Kepler proposed the problem 400 years ago, and a still-controversial proof was only proposed in 1998.
Any traveler who has bought too many souvenirs will recognize the conditions of Kepler’s “bulk” packing problem: The number of units to be packed is virtually unlimited, and space is at a premium. But, as travelers also know, packing strategies may vary, depending on whether objects are to be placed deep within a full suitcase or carried inside a petite handbag.
Similarly, in small spaces with relatively few units, the “orange display” structure for bulk materials may not work best for the items being packed together. Within a small chink in a larger lattice structure, for example, or an isolated complex of particles, a variety of different structures may form instead.
The Science researchers studied this sort of finite packing, experimenting with plastic microspheres inside a shrinking droplet of liquid. The physical principles that drove the spheres’ clustering aren’t fully clear yet, but some of the results don’t look like anything described in nature before. Interestingly, the researchers didn’t set out to discover new laws for assembling matter. What they wanted to do was make materials that could bend light.
Let there be light
In some materials, the arrangement of the molecules, or larger particles called colloids, affects how light bounces around on its way through. Researchers can take advantage of this phenomenon in materials called “photonic crystals,” to transmit information in the form of light, through optical circuits. Pine and his colleagues were looking for new colloid materials that might be useful for fiber-optic circuitry.
Colloids, such as the plastic microspheres that Pine and his colleagues used, are a few nanometers to micrometers in diameter, many times smaller than the width of a human hair. The researchers were initially hoping to create a photonic crystal by coaxing the microspheres to arrange themselves the same way that carbon atoms do in a diamond.
They mixed their microspheres into an oillike liquid, toluene, and emulsified it with water. Then they applied just enough heat to make the toluene droplets evaporate. The spheres condensed as the droplet shrank, like a leaky balloon, and then stuck together as the droplets disappeared altogether.
Every cluster containing a certain number of spheres was shaped the same way — an important sign that the spheres weren’t just clumping together randomly.
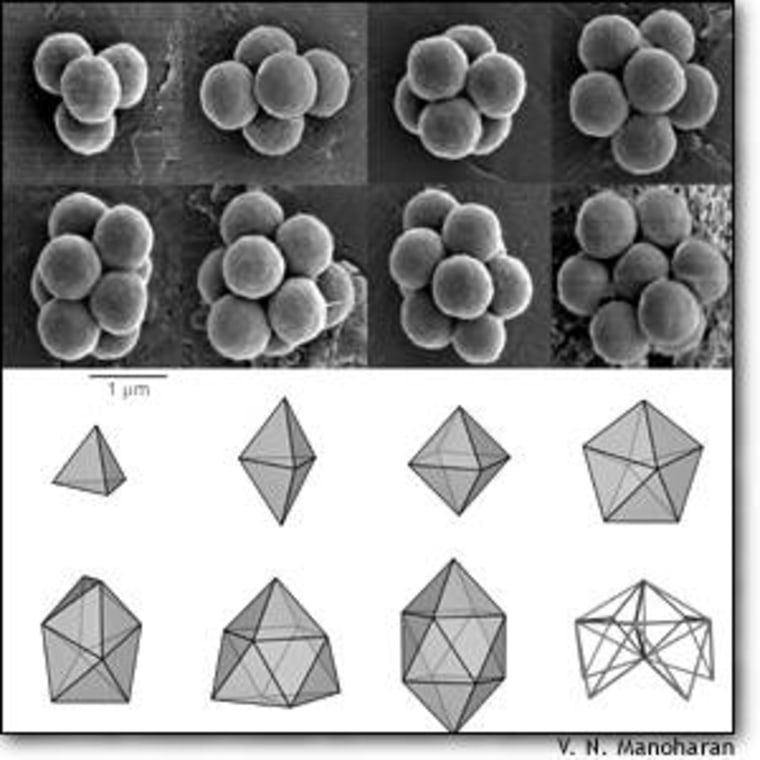
The smaller cluster structures occur in many common molecules, but the larger clusters were far more unusual.
“A lot of these clusters we’d never seen before, although some have been reported in the literature,” Pine said.
The researchers couldn’t fully explain the physical processes that generated the clusters. But, Manoharan discovered a recent mathematics paper suggesting that the different shapes of such clusters are governed by a simple mathematical principle. That is, they minimize the distribution of particle distances from the center of mass of each cluster. Pine found the confluence of the two approaches “absolutely shocking.”
These other authors “weren’t thinking about colloids or emulsions; they were thinking about a mathematics problem,” he said.
“These clusters were weird enough to begin with. And to see them turn up in exactly the same form in a mathematics paper for completely different reasons was just stunning.”
In search of more clusters
Pine and Manoharan are optimistic that the structures they discovered may occur in other environments besides the one in their lab. Pine speculated that other types of particles may cluster when pushed together in a similar fashion — perhaps in piles of sand, for example.
“We think our paper will stimulate people to look for these kinds of clusters where they haven’t thought to look for them before,” Pine said.
“Often when there’s some kind of new discovery it sounds rare at first, but then turns up all over the place. We don’t know yet whether that’s the case here or not.”
The fact that the researchers can produce large quantities of these colloidal structures in pure form bodes well for making highly sought materials, such as photonic crystals that can manipulate a larger spectrum of light, according to a commentary article by Alfons van Blaaderen of Utrecht University that accompanies the Science report.
Further studies of their behavior may shed light on some “fundamental” issues in the physics of solids and liquids, van Blaaderen also noted in his article.
Pine acknowledged that the practical applications of the research were significant, but he’s also been delighted by intellectual experience.
“Every now and then you see some correlation between what mathematics predicts and what nature does. It has a pure aesthetic appeal, and is one of the things that makes doing science so enjoyable,” he said. “It’s really quite beautiful and fun when it happens.”
