If eating were like pumping gas — just fill it up and go — obesity wouldn’t be as prevalent as it is today. As anyone who’s tried to lose weight knows all too well, however, food is far more than fuel to most of us. It’s tied in with our emotions, motivations, and maybe even an ancestral impulse to store energy for leaner times ahead. In a study that could lead to more effective treatments for obesity, researchers have traced brain impulses to show how appetite may be linked to higher-order processes such as emotion and judgment.
In Friday's issue of the journal Science, Jeffrey Friedman of Howard Hughes Medical Institute at The Rockefeller University and his colleagues describe a novel glow-in-the-dark technique that allowed them to see how cells within a mouse’s brain interact to send the “I’m hungry” signal to the rest of the body.
Such insights could help researchers design better drugs to suppress the feelings of hunger for overweight people. Friedman and his team are also optimistic that their method could be used to trace the neural circuitry behind other complex behaviors, such as drug and alcohol addiction.
Leptin and beyond
The body has a sort of fat thermostat, which regulates weight by influencing appetite, metabolism and other functions. Some people may be obese because their “set point” is too high. It’s also possible that obesity is on the rise because the thermostat mechanism evolved in an environment where food wasn’t as readily available as it is today.
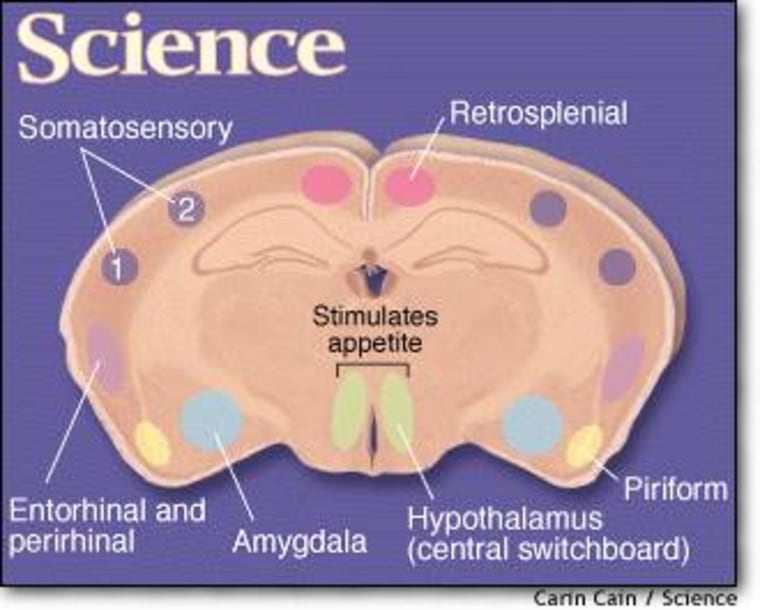
A key component of the fat thermostat is the hormone leptin, which is produced mainly by fat cells and triggers neurons in the hypothalamus, the brain’s central switchboard for regulating conditions inside the body. In general, an increased amount of fat leads to the production of more leptin, and vice versa. A change in leptin levels sparks a set of responses aimed at returning weight to the starting point, such as increasing or decreasing food intake.
When Friedman and other scientists first discovered how leptin regulates weight in mice, their findings raised hopes that the hormone might be a long-awaited obesity treatment for humans.
Researchers’ initial attempts at using leptin to reduce people’s weight met with limited success, however. One likely explanation is that leptin levels, while important, probably aren’t the only factors involved in the decision to start eating.
For their current Science study, Friedman’s team wanted to know whether neurons that responded to leptin’s signal might also be in communication with neurons from other parts of the brain seemingly unrelated to feeding.
“Feeding is a motivational behavior, not a reflex. We wanted to understand more about how the higher order and more basic neural systems talk to each other,” said Friedman.
Such research would require tracking the activity of many chains of neurons at once.
A virus sheds light
To trace the neural circuitry of appetite, Friedman’s team used a modified animal virus that left a fluorescent trail as it infected each neuron in the pathway.
“We created an on/off switch that activates the virus only in the neurons we’re interested in,” Friedman said.
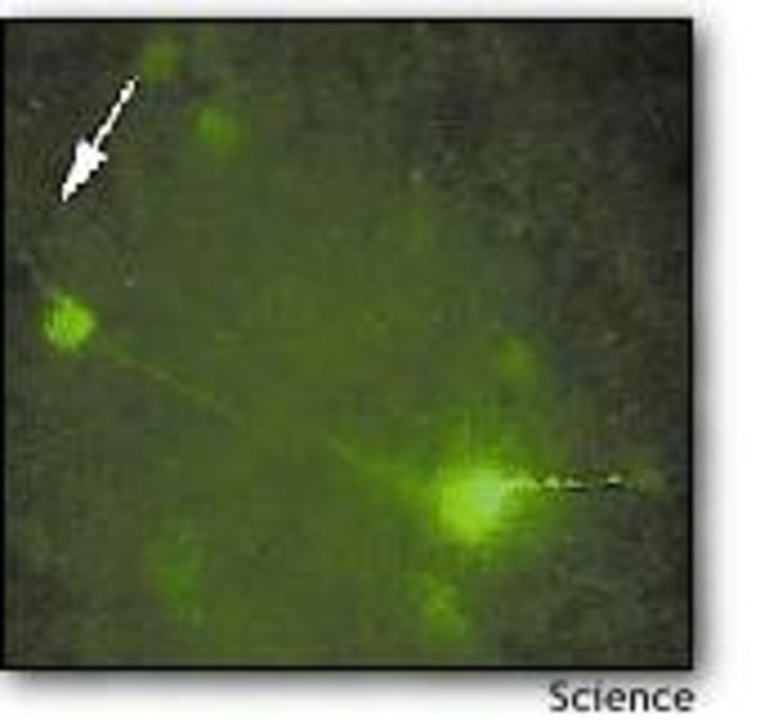
The virus was a harmless type of herpes virus, modified so it could only replicate in neurons producing a certain enzyme. It also produced a green fluorescing protein whenever it infected a new neuron, thereby marking its pathway. In order to study the parts of the brain that directly controls feeding behavior, the researchers injected the virus into the hypothalamus of genetically engineered mice. Only the leptin-receiving neurons in the mice produced the enzyme targeted by the herpes virus, so the activated virus illuminated that particular neural circuit.
Looking at the brain tissue under the microscope, the researchers could see the activated neurons glowing green against a black background. The glow spread over time, as the virus followed the pathways through the brain.
Friedman’s team used the same approach to trace the activity of a second group of neurons, which produce another protein involved in the regulation of appetite. In both cases, they saw a telltale swarm of green dots in a variety of mouse brain regions, such as the amygdala, which controls emotion in humans, and parts of the cortex that humans use for higher-order thinking.
Targets for new therapies?
These findings indicate that the brain’s appetite-regulation system responds to additional signals, besides the simple “we need more energy” message that comes from fat cells. That makes sense, according to a Friedman’s co-author, Jeff DeFalco, also from the Howard Hughes Medical Institute.
“Feeding strategies have developed by evolution. When an animal finds food, it has to decide whether or not to feed. It’s going to take many factors into account, such as its own energy stores, its emotional state, the safety of the environment,” DeFalco said. “Our research suggests that many of the signals are sensed by key neurons in the hypothalamus.”
But what about humans trying to lose weight? DeFalco and Friedman say that blocking or somehow manipulating these additional signals could eventually be the key to suppressing appetite in people who have too much fat.
Friedman’s group now hopes to identify the type of neurotransmitter that’s carrying the additional signals from one neuron to another in the feeding pathway. These molecules may be useful targets for antiobesity drugs.
Another route might be to modify the virus so that it traces the appetite signal after it leaves the leptin circuit and activates other “downstream” neurons. This point in the pathway might be another good place for appetite-suppressing drugs to intervene.
“Successful antiobesity drugs will have deal with the two-way traffic between the higher-order and more basic systems in the brain that work together to tell the body to eat. By illuminating this traffic, our neural tracing method could eventually lead to exciting new avenues for treating obesity, ” DeFalco said.