As an infectious disease specialist, Dr. Warren Dinges is only too aware of looming worries about swine flu this fall.
"It's clearly a threat and a problem," he said.
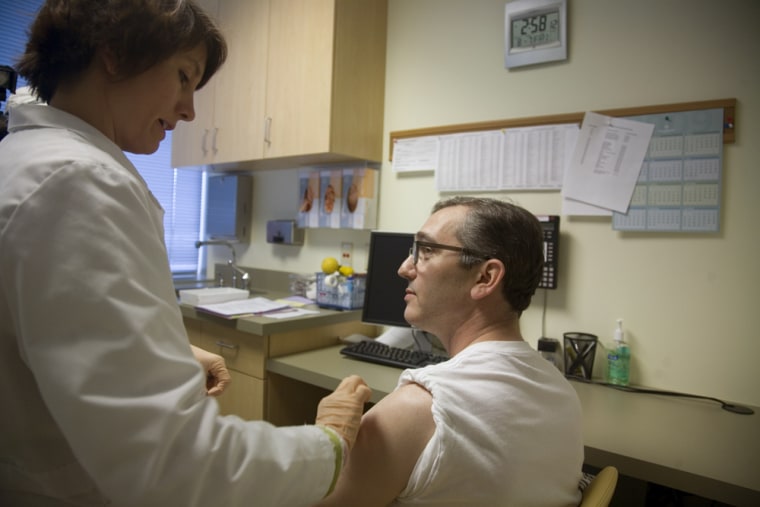
So on Friday, the 41-year-old Seattle internist stripped off his shirt and bared his left arm to become one of the first people in the nation immunized against the novel HIN1 influenza virus.
Dinges was among a handful of people nationwide to receive the first jabs in clinical trials aimed at assessing the safety and effectiveness of a new vaccine to protect against a pandemic strain of H1N1 influenza.
Three people at Group Health Cooperative in Seattle, another five at St. Louis University and six people at the University of Maryland School of Medicine were inoculated against the virus Friday, according to early reports from trial sites.
Vaccines were shipped to eight Vaccine Treatment and Evaluation Units across the nation Thursday night, said Linda Perrett, a spokeswoman for the National Institute of Allergy and Infectious Diseases, which is coordinating the trials.
Rolling out the vaccine
Starting Monday, hundreds of volunteers nationwide will join the fast-track tests that must be completed before nationwide vaccine distribution can begin, probably in mid-October.
As many as 2,800 people are expected to participate in five initial trials of vaccines produced by two manufacturers, Sanofi Pasteur and CSL Biotherapies. Tests on healthy adults ages 18 to 64 will be followed by shots for volunteers older than 65 and children ages 6 months to 17 years.
The main aim of the trials is to to see how much vaccine it takes to elicit a strong immune system response to beat back the new virus, said Dr. Lisa Jackson, the principal researcher who's heading the trials at Group Health Cooperative in Seattle.
"It's very important to see whether we need a higher strength or not," said Jackson.
The trials will test whether one or two shots of vaccine, at both 15-microgram and 30-microgram doses, are necessary to stimulate immune system protection against the virus. Other studies will determine whether it’s safe to give seasonal flu vaccine along with the H1N1 vaccine in adults and children.
The new vaccine is very similar to seasonal flu vaccine, with only the new strain of the virus added. Researchers expect it to be as safe as the regular shots, with only mild side effects such as fever in children or redness at the injection site. Health officials note that there's no danger that the vaccine will induce flu because it contains only parts of the inactivated virus.
Alarming side effects would include fever in adults and a high number of allergic reactions, Jackson said.
Dinges, who is familiar with clinical trials, said he was not the slightest bit concerned about being the first in line for the shot.
"I feel quite confident there won't be any significant side effects," said Dinges, who will return in three weeks for a second H1N1 shot.
Without vaccination, more than half of the U.S. population could become infected by the virus, with more than a third becoming ill, perhaps seriously, according to estimates by leading influenza researchers.
The trials are scheduled to take weeks, not months, with the goal of beating the fall and winter flu season.
If all goes well, U.S. health officials expect to use perhaps 200 million doses of the vaccine, which is aimed at stopping the previously unknown virus that has sparked a global pandemic and killed more than 1,154 people, including at least 436 in the United States. At least 168 countries and territories have reported swine flu, according to the World Health Organization. Cases are popping up so quickly that national and international agencies have stopped counting.
3 jabs likely
U.S. health officials have said they expect that most people would need three flu shots this year: a single jab of seasonal flu vaccine, followed by two doses of swine flu vaccine given 21 days apart. They won't know for sure until the results of the trials are tallied, probably in late September, making any predictions now still speculative. National and local health officials are still working out the complicated logistics of distributing the vaccine.
Supplies of vaccine are expected to be scarce, especially in the early weeks. Health officials have recommended that pregnant women, health care workers and children and young and adults ages 6 months through 24 years be first in line. Parents and other caregivers of infants and non-elderly adults with high-risk medical conditions also are on the list.
The list does not include people older than 65. But officials with the federal Centers for Disease Control and Prevention are urging them, and everyone else, to get shots for seasonal flu as soon as they’re available this fall.
Dinges said he plans to get a seasonal flu shot, too, even though he'll have to wait until after results are in from the second H1N1 injection. But by then, his contribution will have helped determine how protected everyone will be against the return of the new flu next fall.
"I definitely am glad that I can help out," Dinges said.