About 12 million more obese Americans could soon qualify for surgery to implant a small, flexible stomach band designed to help them lose weight by dramatically limiting their food intake.
The FDA will make a final decision on the Lap-Band in the coming months.
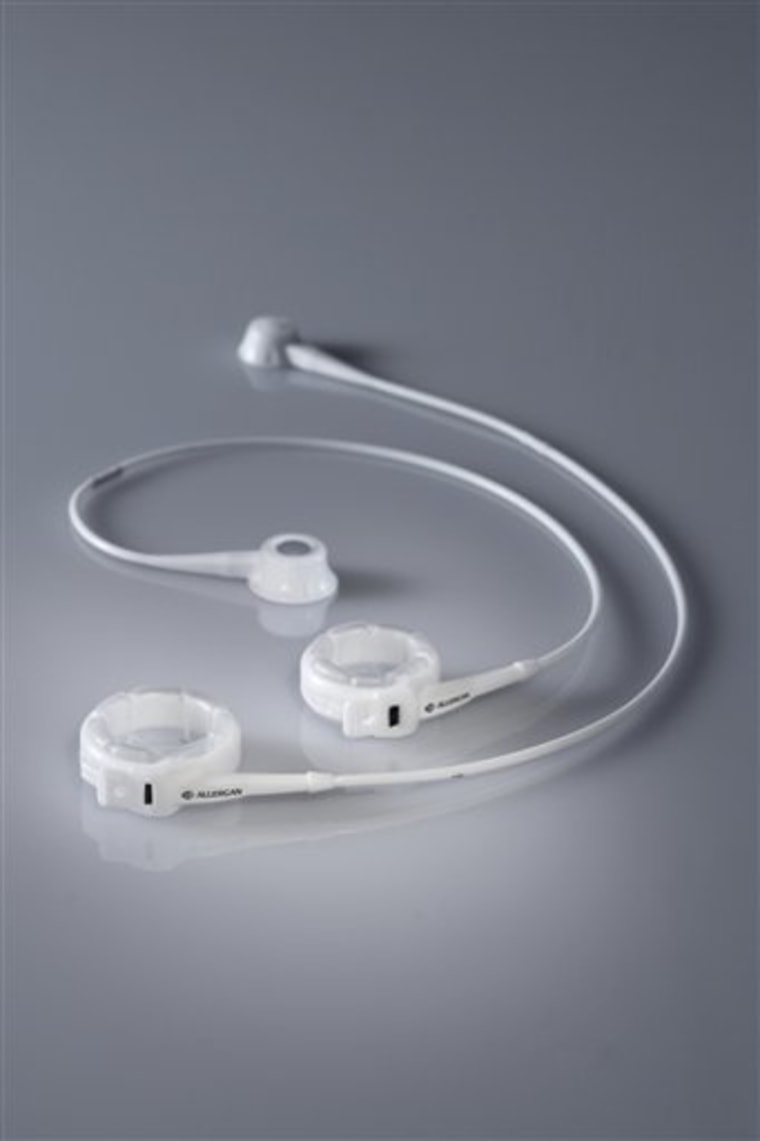
The device from Allergan Inc. is currently implanted in roughly 100,000 people each year and usually helps patients lose 50 pounds or more. Under federal guidelines, it has been limited to patients who are morbidly obese.
On Friday, a panel of Food and Drug Administration advisers recommended expanding use of the device to include patients who are less obese. The panel voted 8-2 that the benefits of broader approval outweighed the risks.
If approved for wider use, the Lap-Band could be available to patients like Angela Denson, a 37-year-old Indianapolis woman who wants to lose 80 or 85 pounds. She said she has struggled with obesity since she started having children 20 years ago.
"I've tried diet pills. I've tried Weight Watchers ... all different types of diet plans," she said.
Denson is not quite obese enough for the surgery under the current standards, but she still wants to pursue the procedure to ward off future health problems and feel better.
But experts stress that the Lap-Band cannot stop deeply ingrained behavior that drives people to overeat. And the high cost of the procedure will remain a barrier for many potential patients.
More than a third of all American adults are obese. About 15 million of them meet criteria for gastric banding surgery under existing guidelines, which say a person should have a body mass index of 40 or higher, or a BMI of 35 or higher if the person suffers from a weight-related medical problem such as diabetes or high blood pressure.
If adopted, the proposal would lower the Lap-Band requirement to a BMI of 35 or higher, or as low as 30 with one related problem.
Doing so would increase the number of eligible patients to 27 million, according to federal health data.
Denson said her insurer denied her doctor's request for a band procedure because her BMI was 39.3, and she had no serious conditions.
Dr. Jack Ditslear said broader approval could help people with lower BMIs avoid dangerous complications down the road.
"We know that being overweight increases the risk of diabetes, high blood pressure and heart disease," said Ditslear, a surgeon at Clarian Bariatrics in Indianapolis. "Ideally you want to lose the weight before you have the onset of those diseases."
The adjustable band has been available in the U.S. since 2001 but far longer in Europe and Australia, where it is dominant. A ring is placed over the top of the stomach and inflated with saline to tighten it and restrict how much food can enter and pass through the stomach.
The device was developed as an alternative to gastric bypass surgery, a permanent procedure in which food is rerouted from a pouch in the stomach to the small intestine.
There were about 220,000 gastric surgeries last year, with banding accounting for an estimated 40 percent. Surgeons say the fact that the procedure is reversible and relatively low-risk accounts for its growing popularity.
"As a clinician, it's pretty common for patients to come in because they've heard about banding," said Dr. Eric DeMaria, a surgeon at Durham Regional Hospital. "It's probably the lowest surgical risk procedure available for morbid obesity."
But there are hurdles to wider use of the procedure, particularly its cost, which can range from $14,000 to $20,000. The device itself costs $3,000.
Susquehanna International analyst Gary Nachman says both insurers and patients are often reluctant to pay.
"It's a very expensive procedure and even if someone has coverage, they may have to pay a copay of a few thousand dollars," Nachman said. "And that's why in a tough economy, we've seen this franchise struggle more than you would normally."
According to Nachman, the payment issues for Lap-Band will only increase if it is approved for patients with less severe obesity. He projects a modest 8 percent rise in Allergan's business through 2014 to about $258 million.
Susan Pisano, a spokeswoman for the industry trade group America's Health Insurance Plans, said she believes a majority of insurers now cover bariatric surgeries.
"They may approach this surgery in a cautious way, but I think there is a broad acknowledgment that there is a place for surgery in the treatment of morbidly obese people," she said, noting that some employers who provide group health coverage choose not cover the procedure in their plans.
The FDA's consideration of the Lap-Band comes as rising health care costs threaten to consume nearly a fifth of the U.S. economy. Obesity-related health care spending is estimated at $147 billion, double the level of a decade ago.
While experts say the Lap-Band can help patients control their weight, it cannot replace healthy lifestyle choices.
"It is a tool to make the lifestyle easier, but not easy. It doesn't help people exercise more or resolve their behavioral issues," said Madelyn Fernstrom, director of the University of Pittsburgh's weight management center. "It's most important for people to understand what it can and can't do."
To change eating behavior, the drug industry has invested billions of dollars to develop weight-loss medications, most of which have not proven effective.
The FDA has rejected two such medications this year alone because of safety concerns.
On Friday the agency issued a lackluster review of a third drug called Contrave, which combines an antidepressant with an anti-addiction drug used to treat alcoholism.
Experts say such drugs have been largely unsuccessful at addressing the main obstacle to weight loss: the brain's fundamental drive to eat enough food to maintain current weight.
Dr. Derek Lowe, a pharmaceutical researcher and blogger, says unless medicine finds a way to address that issue, devices like the Lap-Band will have mixed effectiveness.
"There are certainly people who've had gastric bypass surgery and managed to turn themselves back into their original size by sipping on milkshakes all day," he said.