The highly regarded women’s health chief at the Food and Drug Administration resigned Wednesday in protest of her agency’s refusal to allow over-the-counter sales of emergency contraception.
Assistant Commissioner Susan Wood charged that FDA’s leader overruled his own scientists’ determination that the morning-after pill could safely be sold without a prescription, and stunned his employees last week by instead postponing indefinitely a decision on whether to let that happen.
“There’s fairly widespread concern about FDA’s credibility” among agency veterans as a result, Wood told The Associated Press hours after submitting her resignation Wednesday.
“I have spent the last 15 years working to ensure that science informs good health-policy decisions,” Wood, director of FDA’s Office of Women’s Health, wrote in an e-mail about her departure to agency colleagues. “I can no longer serve as staff when scientific and clinical evidence, fully evaluated and recommended by the professional staff here, has been overruled.”
It was an unprecedented public show of discord for the FDA, and prompted lawmakers to call for congressional hearings into whether the nation’s leading public health agency allowed politics to trump science in determining the fate of the morning-after pill called Plan B.
'Stop playing games'
“It is time for the FDA to stop playing games with the health and well-being of millions of American women,” said Sens. Patty Murray, D-Wash., and Hillary Rodham Clinton, D-N.Y. “Day by day, the public’s confidence in the FDA’s ability to make decisions based on scientific evidence of safety and efficacy is eroding.”
Sen. Michael Enzi, R-Wyo., who heads a Senate health committee that oversees FDA, is considering their request for a hearing, and separately has asked the FDA to explain how and why it reached Friday’s decision, a spokesman said.
Another letter from four House Democrats asks President Bush to issue “a clear directive” to federal agencies that all health-related decisions be based on science.
FDA Commissioner Lester Crawford is out of town, but the agency issued a statement Wednesday saying Wood had helped make “significant strides” in advancing women’s health and that “her decision to leave is unfortunate as we work toward solving the complex policy and regulatory issues related to Plan B.”
The morning-after pill is a high dose of regular birth control that, taken within 72 hours of unprotected sex, can lower the risk of pregnancy by up to 89 percent.
The sooner it’s used, the better it works. But because it can be difficult for women to get a prescription in time, Plan B’s maker has been trying for two years to begin nonprescription sales — and FDA’s latest delay was a surprise: Crawford won Senate confirmation to begin his job this summer only after promising senators to make a final decision by Sept. 1.
Instead, Crawford announced Friday that while over-the-counter sales to women 17 and older would be safe, younger teens would still need a prescription because of concern about whether they could use the drug properly — and that the agency didn’t how know how to enforce an age limit. So he opened the question to public comment for 60 days, but wouldn’t say how soon after that FDA would rule.
Plan B opponents, who consider the drug tantamount to abortion and have intensely lobbied the Bush administration to reject over-the-counter sales, praised Crawford’s move, saying easier access to emergency contraception may encourage teen sex.
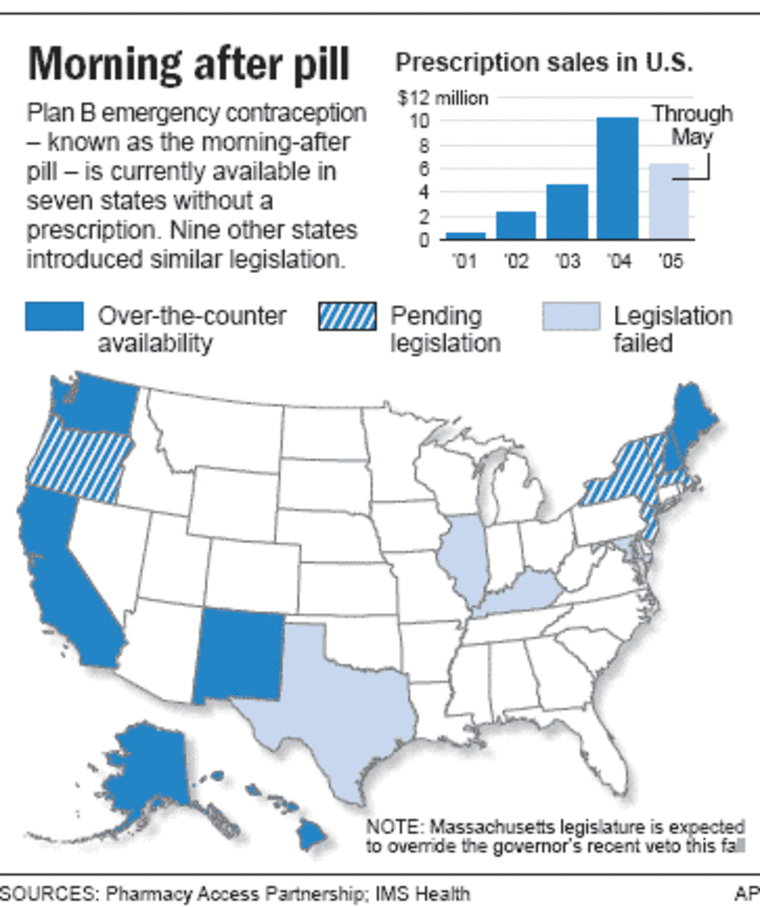
But contraception advocates decried it, saying easier access could halve the nation’s 3 million annual unintended pregnancies. FDA scientists have publicly called the pill safe, used by millions of women with few side effects, and in December 2003 the agency’s scientific advisers overwhelmingly backed over-the-counter sales for all ages.
When FDA first raised the teen concern last year, maker Barr Pharmaceuticals proposed the age limit, saying it could be enforced just like drugstores enforce age limits on cigarette sales.
This time around, Wood said the final decision was made not in FDA’s usual manner but “at the commissioner level ... where most if not all of the professional staff were excluded.” Nor has FDA ever raised questions about teen use of other drugs, she said.
Wood, a biologist, joined FDA’s women’s health office in 2000, after directing women’s health programs at its parent agency, the Department of Health and Human Services. She has worked as a research scientist and a prominent congressional adviser.