A new kind of artificial brain tissue can sustain lab-grown neurons for weeks on end, so well that the neurons respond to hard knocks the way an actual brain would, researchers reported Monday.
The technique could shed new light on the effects of traumatic brain injuries — such as the hits that football players take on the gridiron, or soldiers suffer on the battlefield — and suggest new ways to treat TBI.
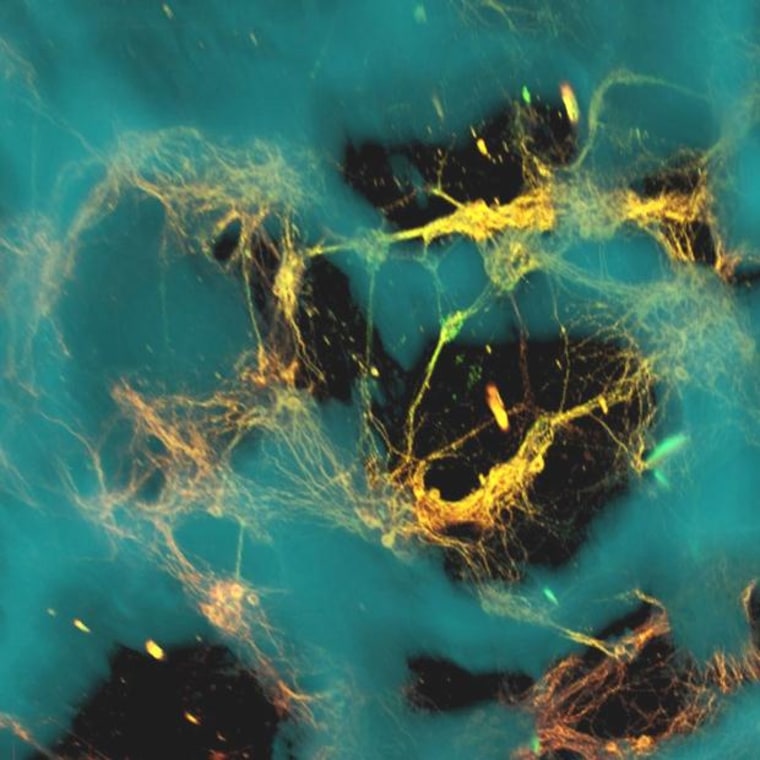
The 3-D tissue is made from a mix of doughnut-shaped rings of spongy silk protein and a collagen-based gel. Thousands of lab-grown rat neurons were "seeded" into each of the protein rings. The rings provided a scaffold for the brain cells, while the gel gave those cells room to send out bundles of fibers known as axons that connected with each other.
"The tissue maintained viability for at least nine weeks — significantly longer than cultures made of collagen or hydrogel alone — and also offered structural support for network connectivity that is crucial for brain activity," Tufts University researcher Min Tang-Schomer, the principal author of the paper appearing in the Proceedings of the National Academy of Sciences, said in a news release.
Lab-grown cells respond to injury
The brain cells that were grown in the stuff passed along electrical signals just as they would in a rat's brain. When the researchers dropped a weight onto the tissue under controlled conditions, they found that the neurons responded much as they would to traumatic brain injury — by releasing high levels of glutamate and sending out spikes of electrical impulses.
The artificial tissue could provide a foundation for new techniques to study how the brain works, and what happens when it's hurt, said senior author David Kaplan, a professor of biomedical engineering at Tufts. "This is perhaps one of the biggest areas of unmet clinical need when you consider the need for new options to understand and treat a wide range of neurological disorders associated with the brain," he said.
In a statement, Rosemarie Hunziker, program director of tissue engineering at the National Institute of Biomedical Imaging and Bioengineering, called the work "an exceptional feat."
The fact that the network of neurons remained active for more than two months — as compared with two or three weeks for other artificial brain tissues — opens up new possibilities for studying how the brain deals with injury and disease, the researchers said.
"You can essentially track the tissue response to traumatic brain injury in real time," Kaplan said. "Most importantly, you can also start to track repair and what happens over longer periods of time."
Computers built with neurons?
Kaplan told NBC News that follow-up work with cultured human brain cells is already under way. "Our key for many of our 3-D tissues is a six-month goal, so we are just at the beginning with the current 3-D brain system," he said in an email.
Could brainlike tissue be used to create brainlike computers constructed with lab-grown neurons rather than silicon chips? "Interesting idea," Kaplan replied. "Not yet pursued, but no reason this could not be considered with the system."
Jennie Close, a scientist at the Allen Institute for Brain Science in Seattle, said the cell-growth technique developed at Tufts appeared to be well-suited for studying traumatic brain injury — but other methods might be preferable for other purposes. For example, she and her colleagues are trying to get brain cells to create their own scaffolding and see how the neurons naturally organize themselves into networks.
"The way that you grow 3-D brain tissue depends on what you want to model," Close told NBC News. "There are a million ways to do this, and I suspect there'll be a million papers describing each approach that can be taken."
In addition to Tang-Schomer and Kaplan, the authors of "Bioengineered Functional Brain-Like Cortical Tissue" include James White, Lee Tien, L. Ian Schmitt, Thomas Valentin, Daniel Graziano, Amy Hopkins, Fiorenzo Omenetto and Philip Haydon. The work was funded by the National Institutes of Health.