Plastics. That was the famously anticlimactic word that failed to captivate Dustin Hoffman in "The Graduate."
And indeed, it's a bit hard to get excited about a science in which the best stuff was invented half a century ago. But a serendipitous accident in an IBM Research lab (also straight out of the movies) may be turning the world of plastics, gels and polymers on its head with a super-strong, super-light, and super-recyclable new material. It may soon find its way into everything from airplane wings to spacecraft.
Polymers, long chains of small, strongly-bonded molecules called monomers, are everywhere, from the kitchen to the International Space Station. But all the cool, unique materials were invented what seems like ages ago: Nylon in the '30s, Styrofoam in the '40s, polypro in the '50s, and so on.
"It's really considered quite a mature field," said James Hedrick, head of IBM's materials lab. The last big jump, he estimated, not counting tweaks to make something less toxic or more flexible, was at General Electric in the early '80s.
That is, until a good old-fashioned "absent-minded professor" lab accident resulted in a new family of materials.
IBM researcher Jeannette Garcia forgot to add a component to a fairly straightforward polymerization reaction, but instead of blending into a toxic slurry, it formed a polymer — and to everyone's surprise, a very strong one.

"I couldn't even get it out of the flask. I had to break the glass with a hammer," Garcia recalls. "The polymer actually survived the glass breaking, so I hit it with the hammer, and it didn't seem to break. I thought, 'Hmm, there could be something to this.'"
What they discovered when they studied the material further astounded them.
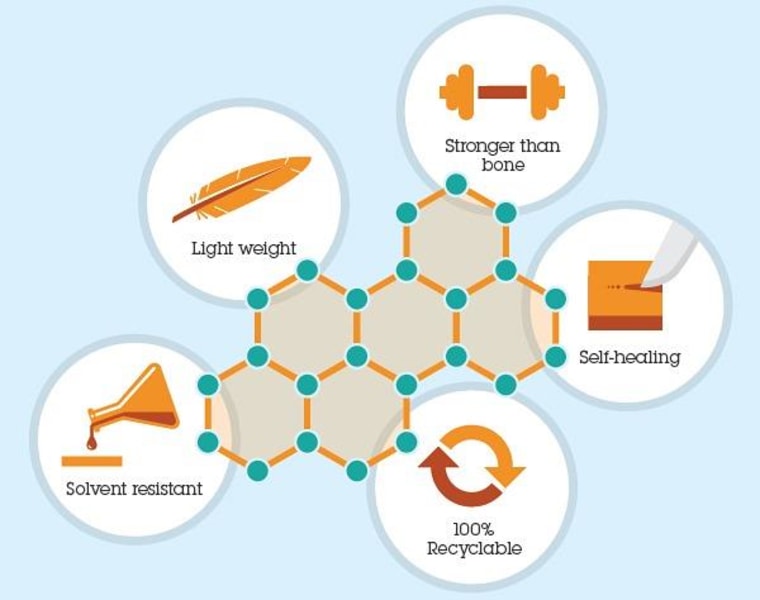
"It's stronger than existing thermosetting plastics by about threefold," explained Garcia. "Even without reinforcement, we're reaching 14 gigapascal's on Young's Modulus."
That's the standard test for strength, by the way: Take a thin slice of a material, be it plastic wrap or aircraft-grade aluminum, suspend it and apply force to the middle, and see when it gives way. Fourteen gigapascals surpasses the score of bone (a benchmark historically) and approaches steel.
Garcia described the material and process in a paper appearing in this week's issue of Science.
IBM's "computational chemistry" techniques also revealed that there was a hidden, stable precursor to the material, meaning it could be further tweaked and added to. Cure the stuff at high temperature and it's rigid enough to serve in aerospace. Cure it at low temperature and you get an "organogel" that sticks to itself, healing gaps and scratches.

But the biggest surprise was yet to come. Both these materials (nicknamed "Titan" and "Hydro" at the lab) can be reduced to their component monomers, the tiny molecules strung together to make the polymers, by incredibly simple methods. Titan disappears in strong acid, and Hydro in ordinary water.
The greatest asset of such super-strong materials is generally also their greatest liability: Nothing affects them.
"They have incredibly great stability — mechanical, chemical, thermal — but it kind of goes in tension with the idea of recyclability," explained Tim Long, a professor of chemistry at Virginia Tech. "If you're going to reduce it back to monomers, that demands that the molecule be unstable."
So for decades, you've had to choose: strength or reusability? The question is not a simple one at, for example, Boeing or NASA, where expensive, high-performance materials must be made to astronomical levels of precision — and if they deviate a tiny bit, or take the slightest damage, they must be discarded.
That's no longer necessary, as IBM has proven. "We can begin as scientists to design molecules that are incredibly tough, incredibly durable, but still recyclable," enthused Long. "Those things don't have to be in tension."
We can have our cake and recycle it too, in other words. Simply showing it's possible may set off a rush of research to make the new, cheaper, more eco-friendly shopping bag or water bottle.
So what can't this wonder material do? Well, you probably won't have it in your kitchen, for one thing. Titan isn't the kind of thing you wrap your extra asparagus in — more like structure reinforcement for a moon lander or the body of a military drone. Hydro's tendency to form little capsules full of whatever fluid it's formed in might find it a spot in cosmetics, but that's still a ways off, too.
What had Long, Hedrick and Garcia excited wasn't the materials they have now, but the ones yet to come. Titan and Hydro aren't just better versions of existing polymers — a slightly lighter kevlar or more transparent PET — they're the first fruits of an entirely new process. In a world where the best methods have been around for the better part of a century, the importance of such a discovery to industry is hard to estimate — but its impact on the Earth's ecosystem may be even greater.
