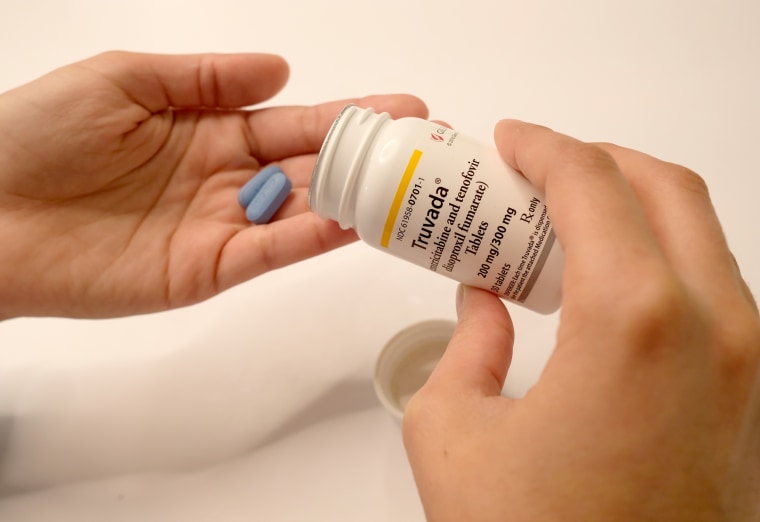
Gilead Sciences will give free Truvada pills to 200,000 uninsured people for the next 11 years to help prevent new HIV infections, the pharmaceutical company said Thursday.
The announcement of the donation came just one day after the company said a generic version of the daily pill would be available in September 2020, a year earlier than expected.
“We are proud to partner with CDC to dramatically expand access to medication that can help prevent new HIV infections,” Gregg Alton, Gilead’s chief patient officer, said in a statement.
Alton said he believes the donation, which will extend through 2030, “can play an important role in ending the HIV epidemic in the United States.” When taken daily, Truvada, the pill used for HIV pre-exposure prophylaxis, or PrEP, prevents HIV transmission.
Health Secretary Alex M. Azar said in a statement that this commitment from Gilead is “a major step in the Trump Administration’s efforts to use the prevention and treatment tools we have to end the HIV epidemic in America by 2030.”
Carl Schmid, the deputy executive director of the AIDS Institute in Washington, and co-chair of the President’s Advisory Council on HIV/AIDS, called the donation “a very significant development” that will “free up the federal government from having to spend potentially billions of dollars over the next 11 years for the purchase of PrEP for the uninsured.”
“After the House Appropriations Committee approval yesterday of an increase of nearly $500 million for domestic HIV programs, today, with this announcement, the Administration’s Ending the HIV Epidemic initiative just received another boost and is now closer to reality,” Schmid said Thursday in a statement.
But some scholars and activists questioned the impact this donation would have on PrEP use, as well as the motive and timing of Gilead’s announcement.
Jen Kates, the director of global health and HIV policy at the Kaiser Family Foundation, a nonprofit health policy think tank, tweeted that if all the 200,000 donated bottles go to people who have never taken PrEP before — which will not necessarily be the case — that would mean that just 36 percent of Americans recommended to take PrEP will be taking it. And that’s only if all of the patients are new.
The Gilead donation also comes on the heels of negotiations between the pharmaceutical company and the U.S. government over potential patent issues, first reported by The Washington Post.
Those negotiations followed an announcement in March by the Global Health Justice Partnership at Yale University that Gilead’s development and testing of Truvada was almost entirely funded by the federal government and therefore the CDC, not Gilead, controls the current patent for PrEP. “Based on our preliminary review, CDC’s Patents for PrEP appear to be valid and enforceable,” the partnership wrote.
Following that news, the HIV/AIDS activist group PrEP4All ramped up its calls for the government to “break the patent” for Truvada and open it up to wider generic manufacturer competition. Outside the United States, where Indian-manufactured generic Truvada is widely available, a month’s supply costs under $100.
The activist group also questioned Gilead’s promise that the free Truvada will be switched to Descovy, a newer, smaller version that potentially has fewer side effects “if it is approved for use as PrEP.” Trials are currently underway to determine Descovy’s efficacy and safety as a once-daily PrEP pill.
“What this is going to allow Gilead to do is essentially transition their entire Truvada PrEP base to Descovy right as Truvada is going off patent, and that's worth a huge amount of money to Gilead,” James Krellenstein of PrEP4All explained.