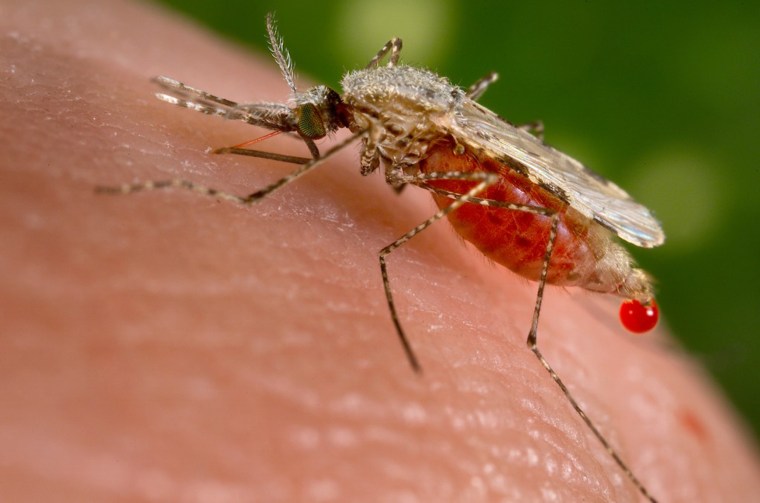
Researchers in California say they have genetically engineered mosquitoes that cannot be infected with the malaria parasite — and they’ve done it in a way that virtually guarantees the trait will spread quickly in a population.
They used a new technique called gene editing, one that allows for the precise placement of new DNA so that it has just the desired effects. And the particular gene editing technique they used, called CRISPR, causes the new trait to spread rapidly because it almost guarantees that the new gene will be passed along to new generations.
“This opens up the real promise that this technique can be adapted for eliminating malaria,” said Anthony James of the University of California Irvine, who helped lead the study.
“We know the gene works. The mosquitoes we created are not the final brand, but we know this technology allows us to efficiently create large populations.”
"The mosquitoes we created are not the final brand, but we know this technology allows us to efficiently create large populations.”
Malaria is a huge problem globally. The parasite infected 198 million people in 2013, according to the World Health Organization. It killed more than 580,000 people, mostly children under the age of 5. There’s a vaccine, but it doesn’t work very well and is not widely used.
The parasite quickly evolves resistance to drugs, so the main tools used to fight malaria are old-fashioned bed nets and pesticides.
Scientists have been working on a number of techniques to make mosquitoes less likely to transmit malaria and other diseases. One big stumbling block is getting the modified mosquitoes to dominate a wild population of mosquitoes.
Valentino Gantz of the University of California San Diego and his colleague Ethan Bier developed a way to get a mutation into both copies of a gene in an insect. That makes it more likely to be passed along.
They combined this with the CRISPR method to get the anti-malaria gene right where they wanted it — a part of the DNA that would be passed along to future generations. This is called germline editing.
So they could tell if their experiment worked, they added a gene that would give the insects glowing red eyes.
It worked. The genetically modified mosquitoes passed the new gene to 99.5 percent of their offspring, they reported in the Proceedings of the National Academy of Sciences.
Now they’ll have to test and see if the mosquitoes that have inherited the new gene actually are incapable of transmitting malaria.
“This breakthrough strategy should also hold promise for many other arthropod transmitted diseases that affect humans and crops and for which the use of insecticides continues to be the main tool,” said Anthony Shelton, an entomologist at Cornell University who was not involved in the study.
Researchers have been trying something less efficient with mosquitoes that spread dengue virus, for instance.
The California team say they doubt their method could ever completely eradicate malaria, but say it could be one of the tools used.