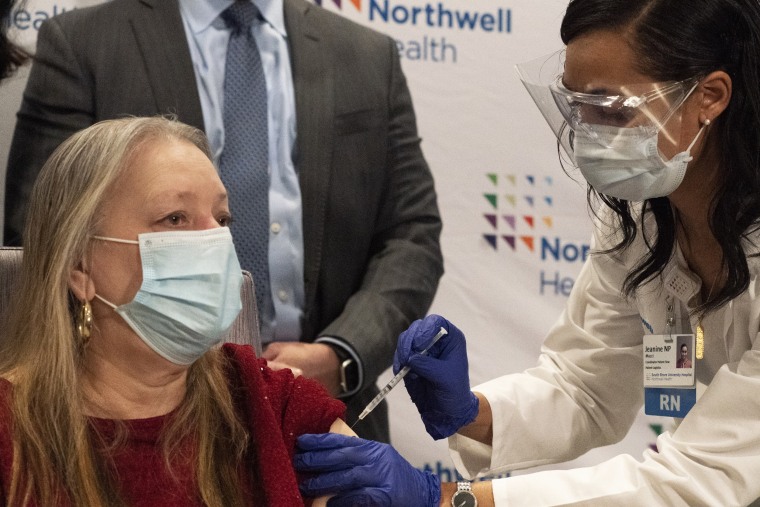
The first 4 million doses of Johnson & Johnson's Covid-19 vaccine are rolling out this week, joining vaccines from the drugmakers Moderna and Pfizer-BioNTech, which have been in use since December.
Johnson & Johnson's single-dose shot, made in partnership with Janssen Pharmaceuticals, differs from the two others in several ways. It's made differently, and, at first glance, it might appear to be less effective.
Full coverage of the coronavirus outbreak
But that doesn't necessarily mean the Johnson & Johnson vaccine is inferior. Here's why:
How effective is the Johnson & Johnson vaccine?
When companies report results from clinical trials, they report the "percent effectiveness" of a vaccine. A vaccine that's 90 percent effective at preventing illness doesn't mean 10 percent of people who received it will get sick; rather, it means people who got the shot were 90 percent less likely to get sick, compared with people who received a placebo.
In Johnson & Johnson's trial, researchers looked at different outcomes in different parts of the world. In the United States, for example, the vaccine was found to be 72 percent effective at preventing what the company defined as moderate to severe Covid-19. That was a broad definition, which ranged from a combination of milder symptoms, such as fever and headache, to more severe symptoms, such as shortness of breath and low oxygen levels.
The effectiveness varied when researchers tested the vaccine in other countries, where variants of the coronavirus are circulating. In Latin America, where the variant P.1 has cropped up, the vaccine was found to be 66 percent effective. In studies in South Africa, where a variant called B.1.351 is circulating, effectiveness was lower: 64 percent.
But the numbers don't tell the whole story.
When researchers looked specifically at the vaccine's protection against the most severe forms of illness, effectiveness shot up to 86 percent.
And it prevented 100 percent of hospitalizations and deaths related to Covid-19. No one who got the Johnson & Johnson shot was hospitalized or died of Covid-19 during the study's follow-up period of 28 days after vaccination.
What's more, the Johnson & Johnson vaccine's effectiveness against severe disease was found to increase over time — to more than 90 percent within a month and a half after vaccination.
What all that means in practice is that even if a vaccinated person is infected with the coronavirus, the illness is likely to be one that can be managed at home, experts say. That's true regardless of the variant.
"Even if they get Covid, their symptoms are much more mild than people who got the placebo and got Covid," said the director of the Alabama Vaccine Research Clinic, Dr. Paul Goepfert, a professor of medicine at the University of Alabama at Birmingham. Goepfert was an investigator in the Johnson & Johnson vaccine trials.
Dr. Paul Offit, a vaccine researcher at Children's Hospital of Philadelphia, put it more bluntly: The Johnson & Johnson vaccine "will definitely keep you out of the hospital, keep you out of the ICU and will keep you out of the morgue."
How does the Johnson & Johnson vaccine compare to other vaccines?
At first glance, the Johnson & Johnson vaccine looks to be the inferior option compared to the vaccines from Pfizer-BioNTech and Moderna, both of which reported around 95 percent effectiveness in their Phase 3 clinical trials.
Experts say that in no way should those percentages be interpreted in a way that suggests that the Johnson & Johnson shot — with its 72 percent effectiveness — is 23 percentage points less effective.
The percentages can't be compared directly to one another, because the trials were run differently. The vaccines were studied at different times during the pandemic, when different variants were circulating.
SARS-CoV-2, the virus that causes Covid-19, has been changing ever since it was discovered in Wuhan, China.
"The virus that swept through China was not the virus that left China," Offit said. "The virus that left China was the first variant, and it was more contagious than the one that originally popped up."
Moderna and Pfizer-BioNTech successfully targeted that variant, called D614G, which was the predominant variant when their trials took place. Johnson & Johnson's Phase 3 clinical trials began later, after the emergence of other variants.
That could account for the decreased effectiveness reported in Johnson & Johnson's trials — there's no way to know how the Moderna and the Pfizer-BioNTech vaccines would have fared in the same scenario.
"I am speculating," said Dr. William Schaffner, an infectious diseases expert at Vanderbilt University Medical Center, "but the notion is that, yes, we think that if the Moderna and Pfizer people had been in South Africa at the same time that Johnson & Johnson were testing their vaccine, we would probably get all very comparable results."
Ultimately, all three vaccines are equally as likely to "keep you out of the doctor's office" if you are infected, said Offit, who was part of the independent panel of experts that voted unanimously to recommend that the Food and Drug Administration authorize the Johnson & Johnson vaccine for emergency use last month.
What are the benefits of this vaccine?
The Johnson & Johnson vaccine can be kept in regular refrigerators, and it doesn't require as stringent cold-storage conditions as the Moderna and the Pfizer-BioNTech vaccines do. That makes it easier to transport and distribute, which could make it a more practical option for mobile or drive-thru vaccination sites and for rural communities.
"It has the potential to reach many people who have had great logistical barriers," said Dr. Muriel Jean-Jacques, an assistant professor of medicine at Northwestern University. "It has literally taken the National Guard and the military to get the vaccine out effectively to many rural communities. How wonderful it is to be able to do that much more simply."
The Johnson & Johnson vaccine also requires only a single dose, which will alleviate the administrative burdens of scheduling two shots several weeks apart. As a result, it could be ideal for communities that have struggled to get the other vaccines.
"I have many homebound seniors who love the idea of having someone come to their home once — or if they have to leave their home, they only have to leave once — and then they're protected," Jean-Jacques said. "So there are many people who would be very happy with a one-shot vaccine."
Dr. Carlos del Rio, executive associate dean of the Emory University School of Medicine in Atlanta, agreed, saying the one-dose regimen might be preferable for some people whose work or home lives may make it more difficult to make two appointments.
Some people "cannot afford to take a day off to get a second vaccine," he said. "If you are unlikely to return for a second vaccine, I think the Johnson & Johnson may be better."
How does the vaccine work?
The Johnson & Johnson vaccine is what's known as an adenovirus vector vaccine. It uses an inactivated adenovirus, a type of virus that can cause the common cold, that has been engineered to carry the genetic code for the coronavirus's spike protein. The genetic instructions allow the body to build the spike protein, which the immune system learns to recognize. That way, if the real virus shows up, the immune system will already know how to defend itself.
The vaccine uses a modified adenovirus to enter cells and deliver the genetic code for the spike protein, but the viral vector itself is harmless.
"It's not a true virus — it has been modified so that you don't get infected," said the director of the stem cell program at the University of California, San Francisco, Dr. Arnold Kriegstein, a professor of neurology.
The vaccines made by Moderna and Pfizer-BioNTech use a different approach to teach the body how to build the coronavirus's spike protein. Both vaccines use synthetic messenger RNA, or mRNA, to deliver bits of genetic code to cells to trigger an immune response.
What's in the vaccine?
After the Johnson & Johnson vaccine was granted emergency use authorization by the FDA, concerns arose about what's in it and how it was made.
The Archdiocese of New Orleans issued a statement Monday urging its parishioners to avoid the vaccine, calling it "morally compromised" because of its links to cells that were derived from tissue from aborted fetuses. Notably, the statement was a departure from the position of the Vatican, which said this year that it was "morally acceptable" for Roman Catholics to receive any Covid-19 vaccine.
The Johnson & Johnson vaccine doesn't contain fetal tissue, but like many other vaccines, it was tested and developed using cells that were derived from fetal tissue decades ago. The cells are commonly used in scientific research because they can replicate easily, which means scientists in effect have an unlimited supply of these "immortalized" cell lines.
"You can grow them very quickly and then they just divide and divide and divide," Kriegstein said.
In vaccine research, human fetal cell lines are used to assess how well the candidates perform. The cells were instrumental, for instance, in the development of vaccines that protect against chickenpox, rubella and polio, Kriegstein said.
"If you're trying to test a vaccine to see if it works effectively and you need to create large numbers of the virus, the quickest way is to grow them in these human cells," he said. "They're perfect hosts for the virus."
Indeed, Johnson & Johnson used cells derived from fetal tissue to grow the adenoviruses needed to manufacture the vaccine. But the cells aren't an ingredient in the vaccines themselves.
"The cells that are used now were never in a fetus — they are not fetal cells," Kriegstein said. "In fact, these cell lines have been used for decades, and they have divided and divided tens of thousands of times, so we're not talking about any cells that actually came directly from a fetus."
What are the side effects?
The most common side effects reported in Johnson & Johnson's clinical trials are generally expected after any vaccination. They include pain at the injection site, redness of the skin, muscle pain, headache, fatigue, nausea and fever. While they may be uncomfortable, they actually signal that the immune system is responding to the vaccine.
"It's better tolerated than the Pfizer or Moderna vaccine in terms of local, what we call reactogenicity — [it] causes less people to have a sore arm, less people have what we call systemic side effects, including fatigue, fever, myalgias [and] headache," Goepfert said.
Download the NBC News app for full coverage of the coronavirus outbreak
No serious or long-term side effects were reported during Johnson & Johnson's clinical trials, but the company is investigating two cases of severe allergic reactions that were reported later. Health care providers are advised to monitor people who have histories of allergic reactions for 30 minutes after vaccination and all other patients for 15 minutes.
According to the Centers for Disease Control and Prevention, rare cases of anaphylaxis, a life-threatening allergic reaction, occurred at a rate of 4.5 cases per 1 million doses administered of both the Moderna and the Pfizer-BioNTech vaccines.
When will it be available?
States already got their first batches of the Johnson & Johnson vaccine this week. In addition to the 4 million doses that shipped out Monday, the company is expected to have delivered a total of 20 million doses by the end of the month.
A deal between Johnson & Johnson and Merck was announced Tuesday to speed production of the single-dose vaccine. As was first reported by The Washington Post, the federal government brokered the partnership between the pharmaceutical giants after Biden administration officials learned that Johnson & Johnson had fallen behind on its manufacturing targets.
Johnson & Johnson CEO Alex Gorsky said Monday on NBC's "TODAY" show that the company is aiming to distribute 100 million shots by the end of June and 1 billion by the end of the year.
Follow NBC HEALTH on Twitter & Facebook.