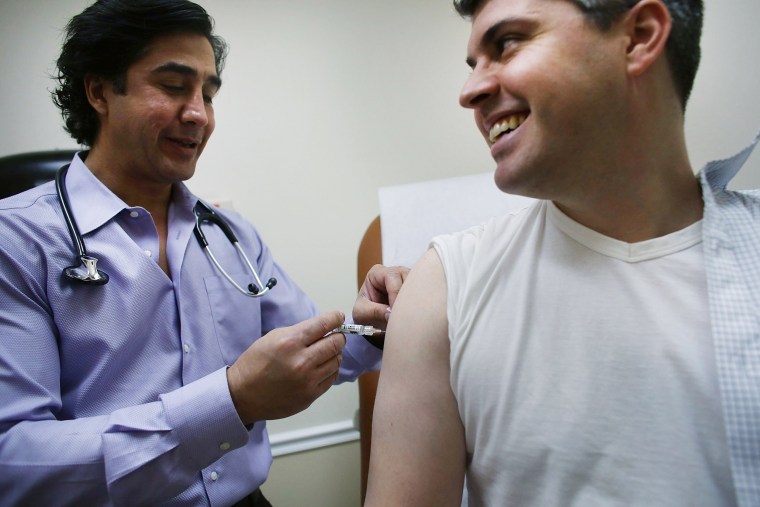
A comprehensive review of 20 years of data finds that U.S. vaccines are remarkably safe, thanks in part to ongoing safety surveillance after vaccines hit the market, according to an Israeli study published Monday in the Annals of Internal Medicine.
"Our study shows that even if a potential vaccine has a rare or long-term side effect that isn't discovered in clinical trials, the surveillance program is in place to identify those issues as soon as possible," said the study's lead author, Dr. Daniel Shepshelovich, vice head of internal medicine at Tel Aviv Sourasky Medical Center.
"Most studies like this show a complicated picture, but here, we saw almost no complexity at all," he said. "Vaccines are safe."
Compared with similar studies Shepshelovich has done on medical interventions, the new study shows vaccines are much safer than many drugs, which can be beneficial but often come with side effects.
"Vaccines have almost no side effects and are especially safer than medical devices," he said.
In the study, researchers used data from the Vaccine Adverse Event Reporting System, or VAERS, a database created in 1990 by the Food and Drug Administration and the Centers for Disease Control and Prevention. The national surveillance system collects about 30,000 reports related to potential vaccine safety concerns every year from health professionals, vaccine manufacturers and the public. The FDA continuously monitors the reports to find patterns or spikes in adverse events reported about a particular vaccine.
The researchers examined post-approval safety issues VAERS picked up in the 57 vaccines the FDA approved from January 1996 through December 2015. The majority of the newly approved vaccines were yearly flu shots. The team found 58 post-market safety-related label changes associated with 25 vaccines and determined the changes to be of limited importance.
Nearly 40 percent of the updated safety guidelines were related to restricting vaccine use in certain groups, such as people who are immunocompromised, pregnant women and preterm infants, which were remedied by not giving them the vaccine. The second most common update, accounting for more than 20 percent of post-market modifications, was related to allergies, almost always to latex packaging.
About 20 percent of safety modifications were related to patients' fainting after receiving the vaccine, although Shepshelovich said that may be due to people who were afraid of needles, rather than a side effect of the vaccine itself. Five percent of the updates actually removed previous warnings and precautions, and just one vaccine, for rotavirus, was recalled, in 1999.
This study really shows once again that vaccines are one of the safest interventions we have.
"This study really shows once again that vaccines are one of the safest interventions we have," said Tara Smith, a professor of epidemiology at Kent State University in Ohio. "The issues they saw were largely correctable just by changing who they give the vaccines to."
Smith, who was not involved with the new research, said the reason some safety concerns are not caught during clinical trials is that phase 3 clinical trials include thousands of people, rather than the millions who receive a vaccine once it's part of standard practice.
"Even the largest clinical trial that we have will still be smaller than the number of people getting the vaccine once it's used in the population," she said. "Post-approval surveillance can show minor issues in certain populations that were missed in the initial clinical trials but are still caught over time."
Indeed, that is why robust safety surveillance programs are necessary, said Dr. Paul Offit, director of the Vaccine Education Center at Children's Hospital of Philadelphia, who also was not involved with the new study.
"The important thing is that you have in place systems to pick up these adverse reactions, and in the U.S., we have several layers of systems that do this," he said. Surveillance programs will be particularly important for the COVID-19 vaccine, he added, because most vaccines take at least 15 years to develop and test in clinical trials, while development of a vaccine for the coronavirus might take as little as 18 months.
Still, Shepshelovich said he believes the FDA will take extra precautions in monitoring for potential adverse effects of a COVID-19 vaccine because the world is paying close attention.
Offit agreed. "I think that the FDA and CDC will be extremely vigilant about following up on possible side effects of the SARS-CoV-2 vaccine, as they are for every vaccine given to children," he said. (SARS-CoV-2 is the name of the coronavirus that causes COVID-19.)
An anti-vaccine movement, however, has found support among some people. Another paper published Monday, in the journal Pediatrics, reported that measles, mumps and rubella vaccination rates in 20 states have dipped below 90 percent. A 95 percent immunization rate is needed to stop the spread of measles.
"I think you should be skeptical of anything that you put in your body. You want to see the data, and you want to make sure that these vaccines are held to a high standard," Offit said. "What you shouldn't be is cynical when there is data that show that the benefits of vaccines outweigh the potential risks. The notion that the FDA or CDC is hiding something isn't even close to the truth."
Shepshelovich said he understands that some people will not accept the findings of his study.
"But if you look at the facts, they are very, very clear," he said. "Vaccines are safe."
Follow NBC HEALTH on Twitter & Facebook.