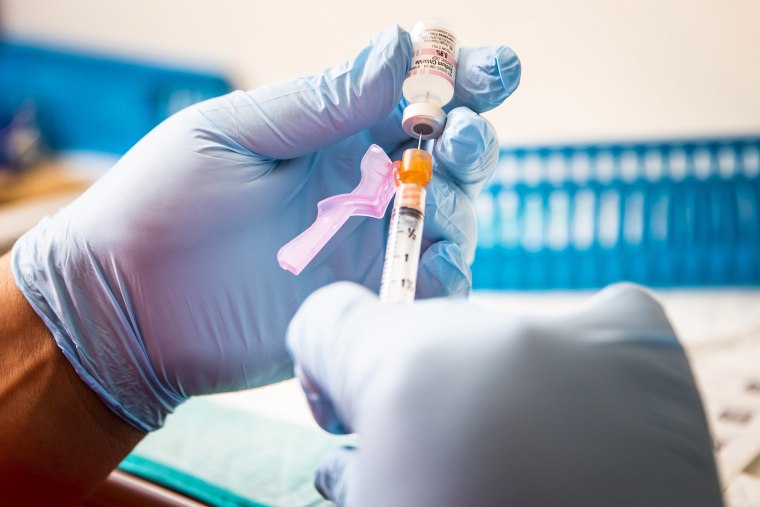
Emergency authorization for Covid-19 vaccines in children under 12 could come in early to midwinter, a Food and Drug Administration official said Thursday, a move that could bring relief to many parents who have been unable to vaccinate their children. The agency hopes to then move quickly to full approval of the vaccine for this age group.
One sticking point for some families who remain hesitant, the official said, is that the vaccines currently in use are administered under emergency use authorization and have not been given full approval by the FDA. Full approval, if it comes quickly after the emergency round, could alleviate that concern.
Full coverage of the Covid-19 pandemic
Covid-19 vaccines have only been authorized for people ages 12 and up in the U.S., and none has received full approval yet.
Both Moderna and Pfizer-BioNTech launched trials of their Covid-19 vaccines for kids under 12 in March. Results are expected in the fall, and it will take FDA officials time to review the drug companies' applications.
The regulatory agency is asking for four to six months of safety follow-up data for kids under age 12, the FDA official said. Just two months of follow-up data was required for the clinical trials in adults.
That additional data could make the process of granting full approval easier. Six months of follow-up data is needed for what is known as a biologics license application, or BLA.
Pfizer and Moderna have applied for full licensure of their vaccine for adults 18 and up. The FDA official said granting full approval for adults is the agency's highest priority.
Pfizer said in a statement to NBC News it anticipates results on its clinical trials in kids ages 5 to 11 sometime in September, and then could apply for emergency use authorization. "Data for kids 2 and under 5 could arrive soon after that," the company said, adding that results on kids ages 6 months up to 2 years may not be released until October or November.
Dr. Buddy Creech, one of the primary researchers for the Moderna KidCOVE clinical trials, which includes children as young as 6 months, predicted a rollout of pediatric data similar to Pfizer's.
"I can't imagine, except maybe for the 6- to 11-year-olds, that we're going to have too much data before the late fall," Creech, also a pediatric infectious disease expert and director of the Vanderbilt Vaccine Research Program at the Vanderbilt University Medical Center in Nashville, Tennessee, said.
Results on kids 5 years old and younger may take longer, he added. "There is still a lot of work left to be done."
Download the NBC News app for full coverage of the Covid-19 pandemic
A safe and effective vaccine for children is an important tool in stopping the spread of Covid-19, especially with the rapid rise of the more transmissible delta variant.
There is no indication that the variant is changing the virus to be more harmful to children. But its highly contagious nature means unvaccinated people, which includes all children under 12, are more vulnerable.
"Given that children are one of the groups that is unvaccinated, we will see more cases in children," said Dr. Richard Besser, a pediatrician and former acting director of the Centers for Disease Control and Prevention. "We will see more hospitalizations in children, and unfortunately we will see more deaths in children."
Besser, who is the current president of the Robert Wood Johnson Foundation, said he hopes the FDA will quickly move to full approval of the vaccines.
"Quite a number of people are saying that that will influence their decision to get vaccinated," he said.
Some pediatric infectious diseases doctors are not convinced full approval, rather than an EUA, would have much of an impact on vaccine hesitancy.
"In terms of how vaccines get approved, there isn't much of a functional difference" between emergency use authorization and full approval, said Dr. Sean O'Leary, vice chair of the Committee on Infectious Diseases for the American Academy of Pediatrics.
As of July 8, more than 4 million children had been diagnosed with Covid-19, representing 14.2 percent of all cases, according to the American Academy of Pediatrics. At least 335 children, ages 17 and younger, have died from Covid-19, according to the latest data from the CDC.
CORRECTION (July 16, 2021, 8:40 a.m. ET): A previous version of this article misstated the number of drugmakers that have applied for full approval from the FDA for their Covid vaccines. It is two, Pfizer and Moderna, not just Pfizer.
Follow NBC HEALTH on Twitter & Facebook.