Last summer, Easton Sinnamon’s parents learned that surgery wouldn’t be enough to fix the leaky valve in his infant heart.
Doctors told them that Easton would need a heart transplant, which would typically mean he would need to remain on immune-suppressing drugs for the rest of his life, so that his body’s immune system didn’t reject the transplanted organ.
But Easton also happened to have another condition that made him a distinct candidate for a novel procedure that, some experts say, could one day have major implications for organ-transplant recipients, by doing away with the need for immune-suppressing drugs.
Easton’s other condition prevented his thymus from functioning properly. The thymus gland is part of the immune system and churns out T-cells, which patrol the body and identify which cells are native to the body and which are outsiders that should be attacked. Typically, when a person receives a transplanted organ, the immune system registers it as an outsider and mounts an attack. Because of that, transplant recipients must take immune-suppressing drugs to tamp down this reaction for their entire lives.
But Easton’s thymus didn’t produce the very cells that would reject a transplanted organ. That detail made him an ideal candidate for an experimental surgery, never performed in humans, in which he would receive both a heart and a thymus transplant from the same donor.
“There’s a chance that by taking [the thymus] from the same donor, the body will recognize the heart as self,” said Dr. Joseph Turek, the chief of pediatric cardiac surgery at Duke University Hospital and a member of the surgical team that performed the landmark procedure.
Turek and his team performed Easton’s heart transplant in August, when he was 6 months old. At the same time, they started to grow cells from the donor’s thymus in the lab. Two weeks later, they removed the infant’s thymus gland and replaced it with the cultured cells that had grown into a new gland in the lab.
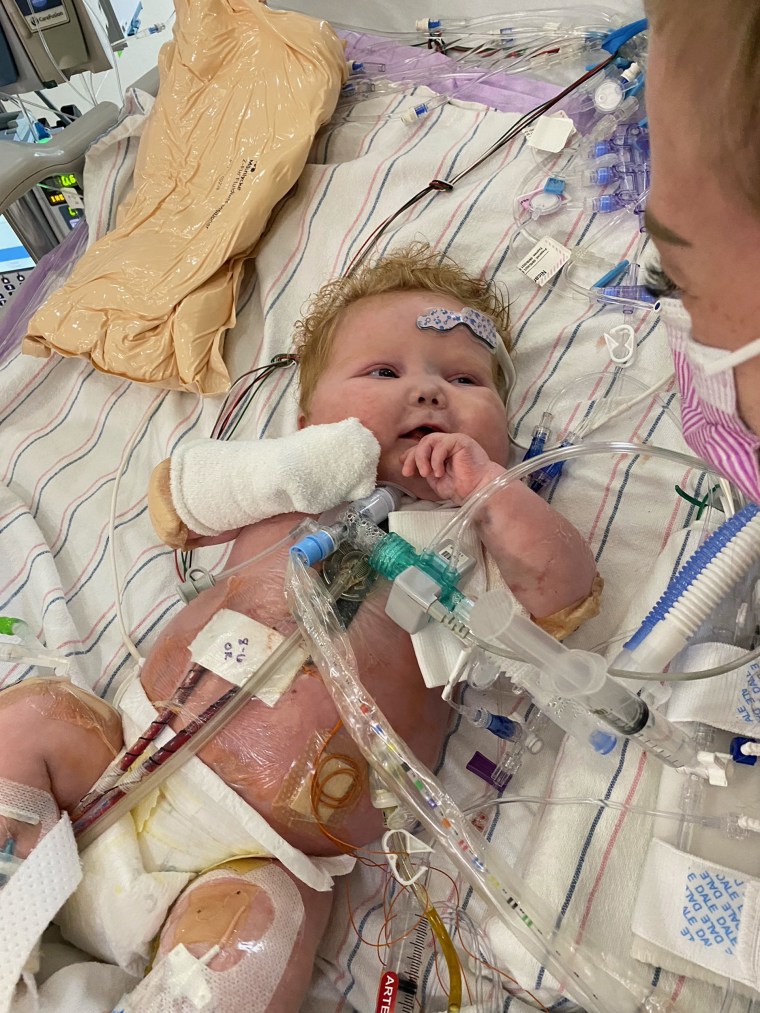
“You can form a brand new immune system, starting from scratch so this new organ can grow up together with that new thymus and be recognized as self,” he said.
The fact that Easton didn’t have a functioning immune system was “vital” to the procedure, Turek added.
If it works and Easton’s immune system does recognize the donor heart as his own, the infant may be able to live without taking immunosuppressants.
Although transplant recipients can often lower their dose of immune-suppressing drugs over time, the medications can be toxic and lead to diabetes, kidney issues and even cancer. The drugs also make people vulnerable to infections, including Covid.
Repeated rejections, which can still occur when people are on immunosuppressants, also put wear on the transplanted organ. For this reason, a donated heart lasts only around 10 to 15 years. But if doctors can figure out a way to make a person’s body accept a donated organ as native, transplanted hearts could last decades.
Tests taken nearly six months after the procedure showed that Easton’s transplanted thymus appears to be producing T-cells. The next phase is to begin to wean him off of the immunosuppressant medication he’s been on since his transplant surgery to see if his body recognizes the donor heart as his own.
According to Dr. William Gibson, a surgical director of cardiac transplantation at Children’s Mercy in Kansas City, Missouri, being able to transplant solid organs — including the heart and kidneys — without the recipient’s body rejecting the donated organ is the “holy grail of transplantation.”
“If this is the first step in being able to do that with a simultaneous thymus transplant, that will potentially change the game in solid organ transplant,” Gibson said.
But even if Easton is able to live without immunosuppressants, it’s unclear whether the procedure would work in someone with a functioning thymus.
“The main issue is we have to try to figure out how to do this in a patient that has a very competent immune system, where you’ll have a native thymus competing with the donor thymus tissue,” Turek said.
If it is feasible, Gibson said that he still expects that it will be a long time before the procedure is available more widely — and some experts question whether it’s possible at all.
Dr. Khadijah Breathett, a heart failure transplant cardiologist at the Indiana University School of Medicine’s Cardiovascular Institute, said that while the thymus plays a big role in immune system function at the beginning of life, during adolescence, bone marrow takes over its duties, including T-cell production.
“The immune system is complex and there are multiple ways the body has to address things that are foreign to it,” she said. “It’s not clear if replacing the thymus will be enough to reduce the need for immunosuppression in adolescents and adults.”
Even in the beginning stages, it’s “outstanding” to see the procedure being done in a human, Breathett said.
Around 75,000 Americans are on a waiting list for an organ donation, but only around 8,000 become available each year, according to the Centers for Disease Control and Prevention.
All people who need an organ transplant face a long waiting list and a serious deficit of donations, but this is especially true for children and babies, Gibson said
“Anything that makes them more tolerated by the recipient would help this problem,” he said.
Follow NBC HEALTH on Twitter & Facebook.
