The acting head of the Food and Drug Administration told his confirmation hearing Tuesday that his decision to revive consideration of over-the-counter sales of the morning-after pill was based on science, not politics.
Dr. Andrew von Eschenbach, President Bush’s nominee to head the agency, was hammered by senators about the timing of Monday’s FDA announcement about the emergency contraceptive pill and why a decision had sat on the back burner for so long.
“We all know what’s going on here. This is a disregard for science out of ideological concerns,” said Sen. Tom Harkin, D-Iowa.
But Von Eschenbach said he made the decision to consider allowing greater access to the pills to women 18 and older, “not on a political ideology, but on a medical ideology.” He said data did not support the safe over-the-counter use by minors.
Federal health officials thought that Monday’s surprise announcement about the pill, known as “Plan B,” would smooth von Eschenbach’s hearing. Instead, he faced sharp questioning from members of the Senate panel.
Sen. Hillary Clinton, D-N.Y., who has put a hold on his nomination until the FDA makes a final decision on the pill, said her hold was intended to “draw a line” against “politicizing the FDA.”
“This is a slippery, dangerous slope we are on, doctor, and we are looking to you to make a decision,” she said.
Sen. Edward Kennedy of Massachusetts, the panel’s ranking Democrat, called the pending Plan B decision “a test case of FDA’s integrity.” If Monday’s announcement “leads to a swift and clear decision, I applaud it,” Kennedy said, “but we must make certain that the administration does not use it as yet another delaying tactic.”
Earlier, von Eschenbach told the panel that under his leadership the agency would be guided by “sound science” and that he was “committed to maintaining the long-standing traditions and values” of the regulatory agency, which has come under fire from some congressional quarters for alleged foot-dragging on approval of the emergency contraceptive pill.
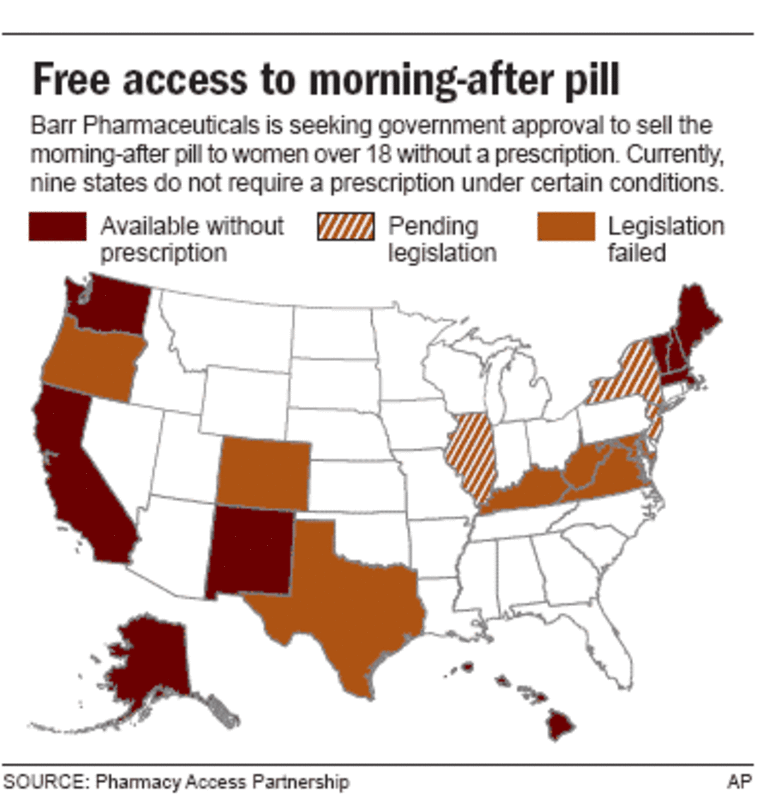
But Clinton and Sen. Patty Murray, D-Wash., renewed their vow to block his nomination until the FDA made a final decision on whether to allow Barr Pharmaceuticals Inc. to sell Plan B over the counter to women 18 and older. Minors would still need a doctor’s prescription.
They removed that hold more than a year ago in exchange for a pledge that the FDA would act on Barr’s application. Crawford won Senate confirmation but then put off a decision on Plan B, earning the enmity of the two lawmakers.
“Fool me once. We are not going to go there again. We will hold this nomination until we have a decision on Plan B,” said Murray, calling the timing of Monday’s announcement “highly suspect behavior.”
Crawford resigned abruptly in September 2005 only two months after the Senate confirmed him to run the agency. Von Eschenbach has been acting FDA commissioner since then. In March, President Bush nominated the urology surgeon to lead the regulatory agency on a full-time basis.
The morning-after pill is a high dose of the most common ingredient in regular birth control pills. When taken within 72 hours of unprotected sex, the two-pill series can lower the risk of pregnancy by up to 89 percent.
Since 2003, the Women’s Capital Corp. and then Barr have sought to loosen the prescription-only restriction on Plan B.
Contraceptive advocates and doctors groups say easier access to Plan B could halve the nation’s 3 million annual unintended pregnancies. Opponents say wider access to the pill could promote promiscuity.
The FDA’s own scientists say the pills are safe, and in December 2003 a panel of independent advisers overwhelmingly backed nonprescription sales for all ages.
The FDA rejected that recommendation, citing concern that young teens could use the pills without a doctor’s supervision. Barr initially had sought approval for over-the-counter sales without age restrictions, but later amended its application to ask for permission to sell to females 16 and older.
In a letter sent Monday to Barr, von Eschenbach said the company should further amend it to limit sales to women 18 and older and create separate packaging to distinguish over-the-counter and prescription versions of the pills. The FDA also wants to discuss Barr’s plans to restrict distribution to certain pharmacies.
“There is no timeline in any statement FDA put out. There is no hint the FDA is any closer to a decision,” Clinton said.
Von Eschenbach has been chief academic officer of the University of M.D. Anderson Cancer Center in Houston and director of the National Cancer Institute. The Philadelphia native has survived three cancer diagnoses: melanoma, prostate cancer and basal cell carcinoma.
