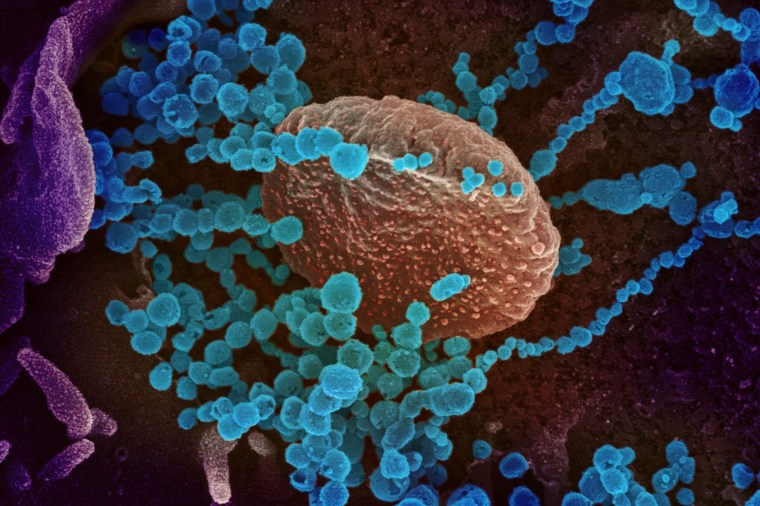
Viruses are among the biggest threats to humanity, with the current pandemic showing how these pathogens can shut down countries, halt entire industries and cause untold human suffering as they spread through communities.
Viruses have also evolved in such a way that they are difficult to kill. What makes them, including the coronavirus, so tricky to cure?
Part of the problem is the nature of viruses themselves. They exist like freeloading zombies — not quite dead, yet certainly not alive.
"Viruses don't really do anything — they're effectively inert until they come into contact with a host cell," said Derek Gatherer, a virologist at Lancaster University in the United Kingdom. "But as soon as that happens, they switch on and come to life."
The odd makeup of these infectious agents is part of what makes them difficult to defeat. Compared to other pathogens, such as bacteria, viruses are minuscule. And because they have none of the hallmarks of living things — a metabolism or the ability to reproduce on their own, for example — they are harder to target with drugs.
"The fact that they are not alive means they don't have to play by the same rules that living things play by," said Britt Glaunsinger, a virologist at the University of California, Berkeley.
Full coverage of the coronavirus outbreak
Antibiotics, which are used to fight bacterial infections, attack the bacteria's cell walls, block protein production and stop bacteria from reproducing. But they aren't effective against viral infections, because viruses don't carry out any of those processes on their own. Rather, viruses need to invade and take over host cells to replicate.
But a virus can't break into just any cell in the body. Instead, one of its proteins will bind to another protein — akin to a key fitting into a lock — which then allows the virus to hijack certain cells. With this outbreak, the coronavirus' so-called spike protein primarily fits "locks" that are present on lung cells, which is why COVID-19, the disease it causes, is mainly a respiratory illness.
Once the invasion takes place, the cell in essence is transformed into a factory that churns out hundreds and hundreds of copies of the virus, based on instructions encoded in its genetic material — RNA, or ribonucleic acid, in the case of the coronavirus.
"It basically acts like a thief inside the cell, stealing all of the cellular machinery and repurposing those machines to make more of the virus," Glaunsinger said.
The human body has evolved defense systems to protect against these kinds of infections.
First, cells have a built-in alarm system to detect viral invaders. The presence of an intruder triggers what's known as an innate immune response, which can involve the host cell releasing a protein that tries to interfere with the virus' replication or can involve the immune system trying to shut down the compromised cells.
But sometimes, these defense mechanisms aren't enough.
"Occasionally if you have a high dose of virus or if a virus has found ways to evade these protective measures, then this innate response can call in reinforcements," said Charles Rice, head of the Laboratory of Virology and Infectious Disease at Rockefeller University in New York City.
The work of these reinforcements to try to defeat the virus is typically what causes the symptoms of a viral infection — in other words, it's at this point when a person may come down with a fever and start to feel sick.
But viruses are sneaky, Glaunsinger said, and they are often able to fly under the radar and cause a lot of damage before any alarms are triggered and any reinforcements are called in. By the time an immune response kicks in, it's often too late.
"At that point, the virus has already been amplifying, it has already been transmitted from that person to other people, and nobody feels terrible yet," she said.
When the immune system is finally triggered, it can also kick into overdrive, causing what's called a cytokine storm, which is thought to be the root of some of the most severe coronavirus cases.
"There's a lot of data coming out that some of the damage might be due to a very strong and brisk immune response, where the body is fighting back and sort of throwing everything it has at the virus," said Dr. Adam Lauring, an associate professor of microbiology and immunology at the University of Michigan in Ann Arbor. "While that may control the virus, it also causes a lot of damage to the lungs."
Download the NBC News app for full coverage and alerts about the coronavirus outbreak
The extreme immune response can worsen pneumonia and cause severe inflammation in the sickest patients, Gatherer said.
The ability of a virus to evade detection is another reason it's difficult to treat with medications.
"The earlier you take the drugs, the better, but by the time someone comes into a clinic, there's already been a lot of growth of the virus, so drugs may slow the virus down, but it may be too late to stop the damage at that point," Lauring said.
Antiviral drugs are also challenging to develop, because they need to work very specifically to combat certain viruses. That's different from antibiotics, which can treat a variety of bacterial infections.
"The features that antibiotics target — the cell wall or the protective coating of bacteria — those things are the same for many different types of bacteria," Glaunsinger said. "That's why you can generate a drug like penicillin that doesn't work against one bacteria but many."
But because viruses hijack human cells, antiviral drugs can't readily target the same features without doing even more damage to the host. Viruses are also more varied, so even pathogens that are closely related — like the current coronavirus and the coronavirus that causes SARS — don't necessarily respond to the same treatments. Similarly, drugs that are used to treat the herpes simplex virus aren't effective against other herpes viruses.
And because viruses have different "keys" to break into cells, there are fewer common targets for drugs to block infections.
"There isn't a common Achilles' heel for all of these viruses," Rice said. "Viruses are just too different."
Even when antiviral drugs are available for some viruses, they don't necessarily cure the infection. Drugs used to treat HIV, for instance, are effective at suppressing virus replication but don't eradicate it. Seasonal influenza can be treated with an antiviral medication called Tamiflu, which can help shorten the duration of the illness, but it's common to be able to detect the virus even after a patient recovers, Lauring said.
Perhaps the only virus that can truly be cured by drugs is hepatitis C, according to Rice, resulting in the virus being eradicated after treatment.
The resilience of viruses is what has made them such a menace throughout history, from flu pandemics to outbreaks of Ebola. And their ability to rapidly evolve, combined with the challenges of developing treatments and cures, will ensure that they remain a significant threat.
"Viruses just want to make more of themselves and find new hosts to infect," Lauring said. "It's truly survival of the fittest."