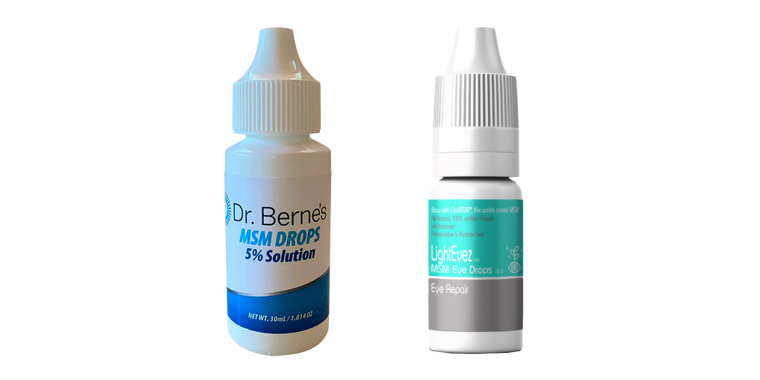
The Food and Drug Administration is urging people to stop using certain brands of eyedrops found to have fungal and bacterial contamination.
The drops in question are Dr. Berne's MSM Drops 5% Solution and LightEyez MSM Eye Drops-Eye Repair. Both are sold online, but are considered illegal because they contain an ingredient called methylsulfonylmethane, or MSM.
The chemical is sometimes used to try to treat arthritis. It is not approved for use in eyedrops, although there are unproven claims online that it can be used for a range of eye problems, including dryness and floaters.
"There are no legally marketed ophthalmic drugs that contain MSM as an active ingredient," the FDA wrote in its warning, dated Aug. 22.
While there have been no reports of injuries associated with using the drops, federal regulators found contamination in samples of the drops that could pose a risk to consumers.
Dr. Berne's MSM drops, according to the FDA, were contaminated with a type of bacterium called Bacillus, and a type of fungus called Exophiala.
The LightEyez drops contained three kinds of bacterial contamination: Pseudomonas, Mycobacterium, Mycolicibacterium and Methylorubrum, the agency said.
"Using contaminated eye drops could result in minor to serious vision-threatening infection which could possibly progress to a life-threatening infection," the FDA wrote.
According to the FDA, Dr. Berne "verbally agreed" to voluntarily recall the product in question. As of Wednesday afternoon, however, those products remained for sale online.
LightEyez, the FDA said, had not responded to the agency.
Authorities are on heightened alert for nonsterile eyedrops after a separate recall earlier this year. The Centers for Disease Control and Prevention identified 81 people in 18 states with Pseudomonas aeruginosa, a type of bacterium resistant to most antibiotics.
Fourteen of those patients were blinded and an additional four were so severely injured that they needed at least one eye removed. Four people ultimately died from their infection.
Most of those patients said they had used a variety or eyedrops, but EzriCare Artificial Tears was the most commonly reported. Those drops have since been recalled, along with Delsam Pharma's Artificial Tears and its Artificial Eye Ointment.
Follow NBC HEALTH on Twitter & Facebook.