The Food and Drug Administration is considering whether it can and should force medical providers to learn more about the best ways to manage pain as part of a “fresh look” in addressing opioid abuse.
The FDA will soon detail the best pain management methods, including those that require opioid use, and support initiatives that ensure that nurses, pharmacists, physician assistants, and other providers get educated about how best to use these powerful drugs, FDA Commissioner Dr. Scott Gottlieb stated Monday.
“We’re actively exploring the question of whether, in the future, there should be mandatory provider education,” Gottlieb said in the opening of an FDA meeting on opioids.
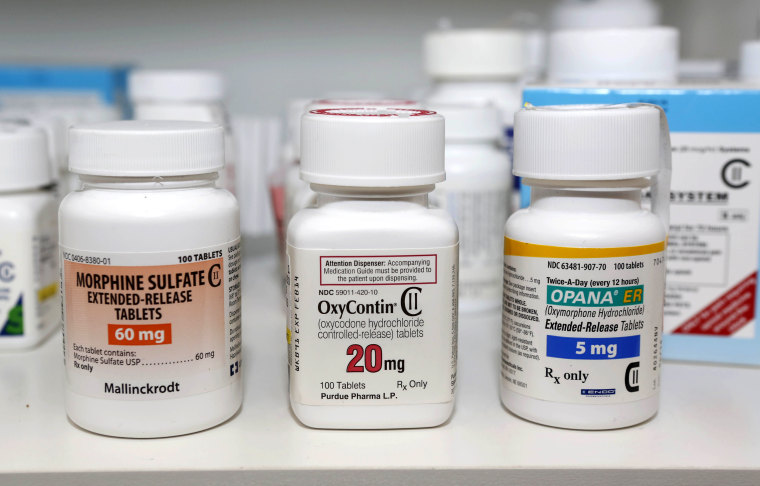
Gottlieb said the practice of over-prescribing opioids has helped drive the ongoing epidemic of opioid abuse. The Centers for Disease Control and Prevention reports that 33,000 people died from opioid overdoses in 2015 and more than 2 million people are addicted to the drugs.
Related: Opioid Prescriptions Are Down But Not Enough, CDC Finds
“I’ve asked my FDA colleagues to take a fresh look at what more we can do to confront this challenge and change the trajectory of the epidemic of addition afflicting our nation. We need to make sure we strike a careful balance between access and safety while taking more vigorous steps to combat the epidemic,” Gottlieb said.
The CDC has been trying to get doctors to prescribe opioids only when absolutely necessary, and to prescribe as low a dose as possible for the shortest time possible.
"America is simply awash in immediate-release opioid products."
“Given what we already know about the scope of current prescribing, and the subsequent patterns of abuse, it’s clear that there should be fewer prescriptions being written for opioids,” Gottlieb told NBC News.
“When opioid prescriptions are written, they should be done so for shorter durations of use. I believe there are still too many 30-day prescriptions being written for conditions like dental procedures or minor surgery, which should require very short-term use, if they require an opioid prescription at all.”
Related: Did This Letter Help Fuel the Opioid Crisis?
The meeting at the FDA Monday and Tuesday is focused on abuse-deterrent formulations of opioids, but Gottlieb said they are not the real problem.
“Most of the exposure to opioid drugs comes from the immediate-release formulations like hydrocodone and acetaminophen or oxycodone and acetaminophen combinations. America is simply awash in immediate-release opioid products,” Gottlieb said.
“In fact, about 90 percent of all opioid prescriptions in the U.S. are written for the immediate-release formulations of these drugs.”
Related: NIH Launches New Push to Fight Opioid Epidemic
Gottlieb said makers of immediate-release opioids will be held to the same requirements as those making the abuse-deterrent and long-acting formulations. That includes making educational materials available to prescribers.
Last month, the FDA asked the maker of one abuse-deterrent pill, Opana, to pull it from the market. After initial resistance, the company complied.
“It’s time to take direct action to address the close to 200 million opioid analgesic prescriptions each year that are for the immediate release products,” Gottlieb said.