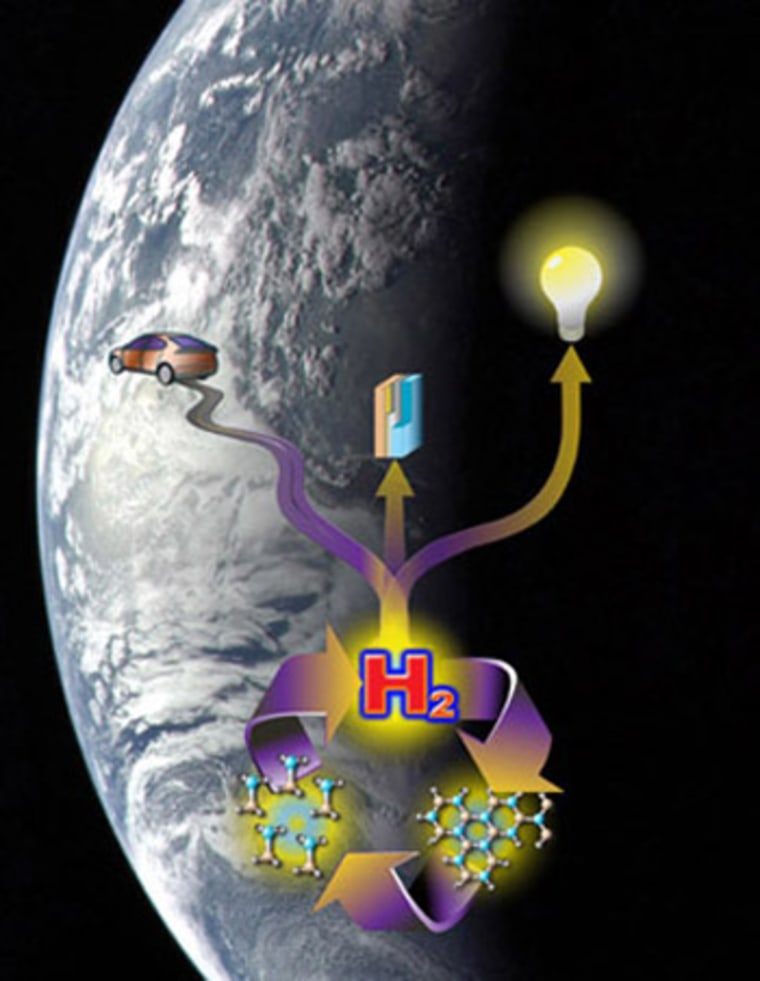
Hydrogen is once again starting to look like a promising green fuel of the future thanks to breakthroughs that permit the fuel to be stored and released from a chemical "tank" that is easily recharged.
The problem with hydrogen, which is easily converted to electricity in a fuel cell and is carbon free, is that it’s a gas that is typically stored in high pressure or cryogenic tanks.
If there's a wreck, the resulting explosion becomes a major safety issue, noted Travis Williams, an assistant professor of Chemistry at the University of Southern California.
"There is also the problem of the best cars we can engineer would probably have a 100 mile driving range per fuel tank," he told me today.
To solve these problems, researchers led by efforts at the U.S. Department of Energy have focused their efforts on a storing the gas in a molecule called ammonia borane, a nitrogen-boron complex that has a hydrogen storage capacity approaching 20 percent by weight.
A block of the stuff could allow a fuel cell car to go 300 miles on a single tank, a benchmark set by the DOE.
The race at the national research labs and academia has been to figure out the best way to take ammonia borane, treat it with a chemical catalyst and get the hydrogen out so it can be used.
"What we've reported is arguably the best way to do that," Williams said of the process he and colleagues report this month in the Journal of the American Chemical Society.
"It works at a mild temperature, the catalyst is reusable, it is air stable, you get a pretty good portion of the hydrogen out and it makes an innocuous byproduct which is intrinsic to the ammonia borane itself," he said.
In addition, the spent fuel can be recharged using a process described by researchers with the Los Alamos National Laboratory this March in the journal Science that uses the chemical hydrazine and liquid ammonia.
The Los Alamos researchers envision an ammonia borane fuel tank that is used to fuel cars, then taken out and sent to a factory to be recharged.
For now, Williams said, using ammonia borane as a hydrogen fuel storage tank would work best for applications such as powering a laptop computer.
With a bit of the catalyst, he said, "it could power something the size of your laptop for a long time and be a lot less heavy than the battery because it is boron and nitrogen, not heavy metals."
This would be ideal, as an example, for military personnel out in the field, who currently carry around heavy batteries to power all their high-tech gadgets.
Getting the process running smoothly and efficiently enough to power hydrogen fuel cells in our cars is at least several years out, Williams noted.
"But I think in our lifetime this is going to become practical," he said.
"The way I see the race is there is going to be a point when we run out of petrochemicals…we are not there yet generally, but we want to have the chemical conversion technology ready so we don't disrupt the economy when that happens."
More stories on hydrogen fuel storage:
- Hydrogen tries to catch up in green car race
- Chicken feathers could store fuel
- Nanocluster acts as hydrogen supersponge
- Fuel cells in your future
- LA gas station gets hydrogen fuel pump
John Roach is a contributing writer for msnbc.com.