Sander Brus didn't expect a photo of his friend's grandfather's flu diagnosis to go viral. But when he shared the image on Twitter in December, he was bombarded with messages, he said.
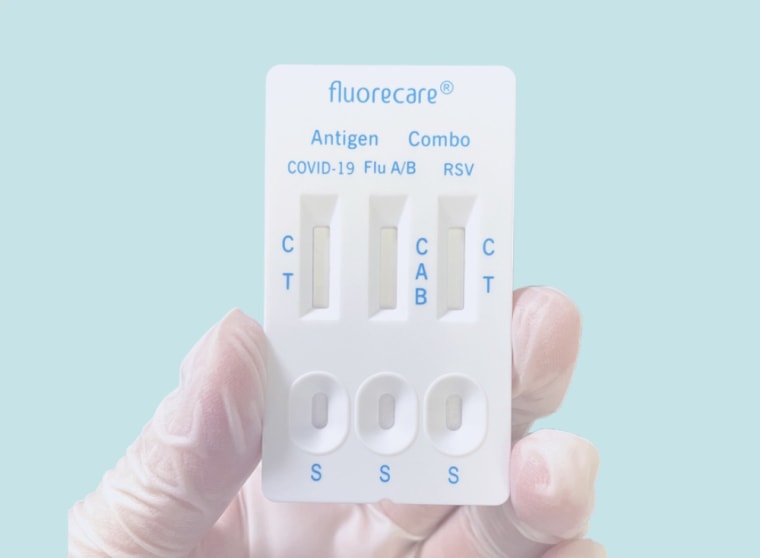
People in the U.S. wanted to know how to purchase the rapid test in the picture, which simultaneously screened people for three common respiratory viruses: Covid, respiratory syncytial virus (RSV) and two types of influenza, A and B.
The viruses have circulated widely this winter and share many of the same symptoms, meaning they're often indistinguishable without a diagnostic test. But unlike Covid tests, which are available over the counter, RSV and flu tests must be performed at a doctor’s office or ordered via prescription. And the FDA hasn't authorized an at-home test that can rapidly detect all three viruses at once.
The rapid test from Brus' photo, however, is available in the European Union, and the manufacturer has certified that it meets the E.U.'s health and safety standards.
Brus is the founder of Measie, a diagnostics vendor that sells the test online. The test costs 6.5 euros ($6.92), and the company has sold around 10,000 of them thus far, Brus said.
The test’s manufacturer, Shenzhen Microprofit Biotech Co. Ltd., says it captures 100% of cases that are negative for Covid, flu or RSV — meaning it won't produce false positives — and picks up on 90% of influenza B cases, 92% of influenza A cases, 93% of Covid cases and 95% of RSV cases.
Brus said that that performance should be sufficient to secure authorization in the U.S.
“A test performed in Amsterdam, where I’m from, will give exactly the same result as a test performed in New York. There shouldn’t be a difference based on geography," he said. "But still, people in the U.S. cannot use it and I can."
Sheng Tang, the vice general manager at Shenzhen Microprofit Biotech, said the company has no plans to apply for emergency authorization for its test in the U.S. Tang said in an email that he doesn't think the Food and Drug Administration would grant the authorization, given its regulatory constraints.
Dr. Susan Butler-Wu, an associate professor of clinical pathology at the University of Southern California, said rapid tests for multiple viruses are "the way of the future," and would help more people get access to the appropriate treatments. But the U.S. has historically fallen behind Europe in terms of authorizing new diagnostics, she said.
“It’s not unusual to see things that have been approved in other countries that are great products and we don’t have them here. That’s just the reality of the situation," she said. "It takes time to go through the process and get approval."
The FDA has authorized one nonprescription test that allows people to simultaneously swab themselves for flu, Covid and RSV, but the swab must be mailed to Labcorp. The test costs $169 for people without insurance.
The FDA said it strongly supports at-home tests for respiratory viruses, including combination tests for flu and Covid. But the agency declined to comment on whether it had received or was evaluating any applications for these tests from manufacturers.
"When the data are sound, the FDA has not hesitated to move quickly to authorize tests, with at-home tests of all areas being a high priority for the agency," an FDA spokesperson said.
The agency pointed to one roadblock, though: At-home flu tests have traditionally required people to swab high up in their nose, which usually means that a clinician should administer them.
Unlike Covid tests, Butler-Wu said, there have been fewer opportunities to study whether rapid flu tests can be administered in the shallower part of the nose and maintain their accuracy.
"You don’t want to have people just touch the outside of their nose and not actually get the benefit of the test," said Joshua Sharfstein, a former FDA deputy commissioner, now a professor of health policy and management at Johns Hopkins University.
Dr. Michael Mina, the chief science officer for the at-home testing company eMed, said the FDA tends to have strict requirements for over-the-counter tests. The agency often asks manufacturers to conduct studies that demonstrate that people can administer at-home tests properly — a process that may cost millions of dollars and delay the test’s authorization by months or years, Mina said.
“It’s taken a very long time in the past to get new self-tests authorized, like HIV tests or even pregnancy tests," he said. "They’ve taken years and years and years and years. We have a pretty conservative regulatory approach."
In real life, Mina isn't worried about combo tests for respiratory viruses being user-friendly.
“We know that Americans can swab their nose because it’s been done millions and millions and millions and millions of times now for Covid," he said.
Even if a doctor administers a rapid test, however, the FDA may still have reservations about its accuracy. Butler-Wu said rapid flu tests given by medical professionals are generally about 60% sensitive, meaning they produce false negatives about 40% of the time. For RSV, that performance is "a little bit better," but still unreliable, she said.
“There are longstanding issues with the performance of antigen testing for flu and for RSV that have been extremely well documented," Butler-Wu said. "In 2009, when novel H1N1 emerged, the sensitivity of antigen tests for flu A was the same as a flip of a coin. We’re talking 50%."
As long as a test is accurate and easy to use, however, the FDA's data requirements "are not that onerous," Sharfstein said. The agency is usually able to make a decision about a test within months of an application being submitted, he added.
“Tests with high sensitivity and specificity will not have difficulty getting cleared by the FDA, but it is important for the FDA to understand how that was determined, what the procedures were, and to actually look at the data,” Sharfstein said.
Mina said he's hopeful that the FDA will authorize a combined at-home Covid and flu test this month or next, but it might not look like the version in Europe. The test could still involve two separate swabs — one for each virus — that are packaged together, he said.
"The FDA is a little bit less comfortable with getting that totally combined test into people’s homes right away," Mina said. "I don’t really understand that part of it. I don’t think there’s good logic behind it.”
But public-health experts haven't given up hope that a combination rapid test for Covid, the flu and RSV will reach consumers one day.
The FDA is "feeling a lot of pressure from Americans," Mina said. "Americans and are now seeing Europeans having these tests available and, just like in Covid, there’s now a push to say, 'Why don’t we have these? Why aren’t they available to us?' The agency is starting to understand that and actually be somewhat amenable to working with the manufacturers to see them get it through.”